6-Bromooxindole
Modify Date: 2025-08-25 18:15:28
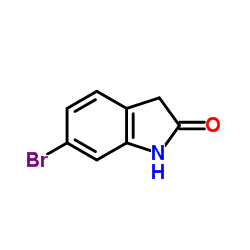
6-Bromooxindole structure
|
Common Name | 6-Bromooxindole | ||
|---|---|---|---|---|
| CAS Number | 99365-40-9 | Molecular Weight | 212.04 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 343.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C8H6BrNO | Melting Point | 217-221 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 161.6±27.9 °C | |
Use of 6-Bromooxindole6-Bromo-2-oxindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 6-Bromooxindole |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Bromo-2-oxindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 6-Bromo-2-oxindole 是一种从海鞘 (D. skoogi) 中分离出来的次级代谢产物。它对 MDA-MB-231 乳腺癌细胞具有细胞毒性,IC50 值为 74.41 μM。6-Bromo-2-oxindole 已用于合成 1,3-distributed 2-oxindoles 和 indolin-2-one p38α 抑制剂,它们具有抗炎活性。 |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 343.6±42.0 °C at 760 mmHg |
| Melting Point | 217-221 °C(lit.) |
| Molecular Formula | C8H6BrNO |
| Molecular Weight | 212.04 |
| Flash Point | 161.6±27.9 °C |
| Exact Mass | 210.963272 |
| PSA | 29.10000 |
| LogP | 2.39 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.625 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933790090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
Synthesis and biological evaluation of new pyridone-annelated isoindigos as anti-proliferative agents.
Molecules 19(9) , 13076-92, (2014) A selected set of substituted pyridone-annelated isoindigos 3a-f has been synthesized via interaction of 5- and 6-substituted oxindoles 2a-f with 6-ethyl-1,2,9-trioxopyrrolo[3,2-f]quinoline-8-carboxyl... |
| 6-Bromo-1,3-dihydro-2H-indol-2-one |
| 2H-Indol-2-one, 6-bromo-1,3-dihydro- |
| 6-Bromooxindole |
| MFCD02179605 |
 CAS#:6127-11-3
CAS#:6127-11-3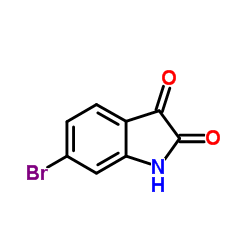 CAS#:6326-79-0
CAS#:6326-79-0 CAS#:7440-66-6
CAS#:7440-66-6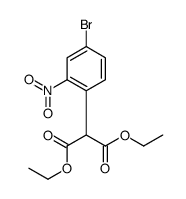 CAS#:199328-34-2
CAS#:199328-34-2 CAS#:100487-81-8
CAS#:100487-81-8![methyl {2-[(anilinocarbonyl)amino]-4-bromophenyl}acetate Structure](https://image.chemsrc.com/caspic/325/1355049-18-1.png) CAS#:1355049-18-1
CAS#:1355049-18-1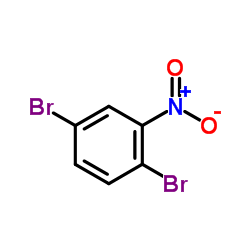 CAS#:3460-18-2
CAS#:3460-18-2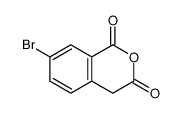 CAS#:552333-33-2
CAS#:552333-33-2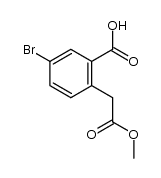 CAS#:1355049-15-8
CAS#:1355049-15-8![methyl [2-(azidocarbonyl)-4-bromophenyl]acetate Structure](https://image.chemsrc.com/caspic/411/1355049-16-9.png) CAS#:1355049-16-9
CAS#:1355049-16-9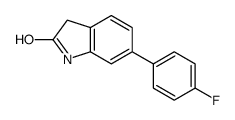 CAS#:258831-98-0
CAS#:258831-98-0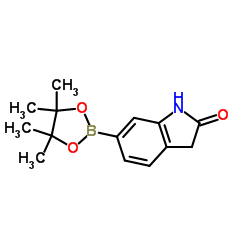 CAS#:893441-85-5
CAS#:893441-85-5 CAS#:63839-24-7
CAS#:63839-24-7 CAS#:199327-63-4
CAS#:199327-63-4![6'-Bromo-1',2'-dihydrospiro[cyclohexane-1,3'-indole]-2'-one structure](https://image.chemsrc.com/caspic/097/1190866-02-4.png) CAS#:1190866-02-4
CAS#:1190866-02-4![6-Bromo-2',3',5',6'-tetrahydrospiro[indoline-3,4'-pyran]-2-one structure](https://image.chemsrc.com/caspic/471/1190861-43-8.png) CAS#:1190861-43-8
CAS#:1190861-43-8![tert-butyl 6-bromo-2-oxospiro[1H-indole-3,4'-piperidine]-1'-carboxylate structure](https://image.chemsrc.com/caspic/297/1160247-29-9.png) CAS#:1160247-29-9
CAS#:1160247-29-9 CAS#:158326-84-2
CAS#:158326-84-2 CAS#:848649-94-5
CAS#:848649-94-5 CAS#:90751-00-1
CAS#:90751-00-1
