L-(+)-古洛糖
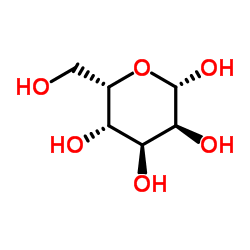
L-(+)-古洛糖结构式

|
常用名 | L-(+)-古洛糖 | 英文名 | L-Gulose |
|---|---|---|---|---|
| CAS号 | 6027-89-0 | 分子量 | 180.156 | |
| 密度 | 1.7±0.1 g/cm3 | 沸点 | 410.8±45.0 °C at 760 mmHg | |
| 分子式 | C6H12O6 | 熔点 | 132ºC | |
| MSDS | 美版 | 闪点 | 202.2±28.7 °C |
|
Preparation of D-gulose from disaccharide lactitol using microbial and chemical methods.
Biosci. Biotechnol. Biochem. 77(2) , 253-8, (2013) When an M31 strain of Agrobacterium tumefaciens was grown in a mineral salt medium at 30 °C containing 1.0% lactitol as sole carbon source, a keto-sugar was efficiently accumulated in the supernatant. This oxidation from lactitol to the keto-sugar was caused ... |
|
|
The role of the gulose-mannose part of bleomycin in activation of iron-molecular oxygen complexes.
Biochem. J. 253(2) , 497-504, (1988) A comparison of the complexing properties of metal ions and O2 activation by bleomycin-A2 (BLM-A2) and deglyco-BLM-A2 is presented. Deglyco-BLM-A2 is obtained from the parent derivative by HF cleavage of the sugar moiety followed by h.p.l.c. purification. Com... |
|
|
Regio- and stereo-selective synthesis of carbohydrate isoxazolidines by 1,3-dipolar cycloaddition of nitrones to 5,6-dideoxy-1,2-O-isopropylidene- alpha-D-xylo-hex-5-enofuranose.
Carbohydr. Res. 226(1) , 49-56, (1992) The synthesis of 2-phenyl-3-aryl and 2-phenyl-3-aroyl derivatives 5-(1,2-O-isopropylidene-alpha-D-xylo-tetrofuranos-4-yl)isoxazolidi ne (3) from nitrones and 5,6-dideoxy-1,2-O-isopropylidene-alpha-D-xylo-hex-5- enofuranose (1) is described. The 1,3-dipolar cy... |
|
|
Microbial production of L-ascorbic acid from D-sorbitol, L-sorbose, L-gulose, and L-sorbosone by Ketogulonicigenium vulgare DSM 4025.
Biosci. Biotechnol. Biochem. 69(3) , 659-62, (2005) Ketogulonicigenium vulgare DSM 4025, known as a 2-keto-L-gulonic acid producing strain from L-sorbose via L-sorbosone, surprisingly produced L-ascorbic acid from D-sorbitol, L-sorbose, L-gulose, and L-sorbosone as the substrate under a growing or resting cond... |
|
|
Stereoselective vinylogous Mannich reaction of 2-trimethylsilyloxyfuran with N-gulosyl nitrones.
Org. Biomol. Chem. 9(21) , 7411-9, (2011) Stereoselective vinylogous Mannich reaction of 2-trimethylsilyloxyfuran with L-gulose-derived chiral nitrones in the presence of a catalytic amount of trimethylsilyl trifluoromethanesulfonate was investigated. The selectivity was strongly influenced by the bu... |
|
|
Gulose as a constituent of a glycoprotein.
FEBS Lett. 298(1) , 14-6, (1992) The aldohexose gulose was identified as a constituent of a hydroxyproline-rich glycopeptide derived from the glycoprotein SSG 185. This glycoprotein is part of the extracellular matrix of the green alga Volvox carteri. The gulose residue occupies a terminal p... |
|
|
The Wittig-cyclization procedure: acid promoted intramolecular formation of 3-C-branched-chain 3,6-anhydro furano sugars via 2'-oxopropylene derivatives.
Carbohydr. Res. 342(8) , 1091-5, (2007) Some olefinic Wittig products, 3-deoxy-5,6-O-isopropylidene-3-C-(2'-oxopropylene)-1,2-O-alkylidene hexofuranose derivatives were converted to the branched-chain 3,6-anhydro-3-C-(2'-oxopropyl) derivatives on treatment with ion exchange resin Amberlite 120 (H(+... |
|
|
HPLC separation of all aldopentoses and aldohexoses on an anion-exchange stationary phase prepared from polystyrene-based copolymer and diamine: the effect of NaOH eluent concentration.
Molecules 16(7) , 5905-15, (2011) To investigate the separations of all aldopentoses (ribose, arabinose, xylose and lyxose) and aldohexoses (glucose, galactose, allose, altrose, mannose, gulose, idose and talose) on the D₆ stationary phase prepared by the reaction of chloromethylated styrene-... |
|
|
Calcium alginate dressings--I. Physico-chemical characterization and effect of sterilization.
J. Biomater. Sci. Polym. Ed. 9(2) , 189-204, (1998) In order to analyze the alginate components of alginate dressings and the fractions which are released when the dressing is in contact with model biological fluids, the use of various analytical methods was considered. The first step was the conversion of a c... |
|
|
GDP-mannose 3',5'-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants.
J. Biol. Chem. 278 , 47483-47490, (2003) Despite its importance for agriculture, bioindustry, and nutrition, the fundamental process of L-ascorbic acid (vitamin C) biosynthesis in plants is not completely elucidated, and little is known about its regulation. The recently identified GDP-Man 3',5'-epi... |