N(α)-Boc-L-2,3-二氨丙酸
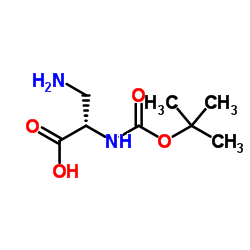
N(α)-Boc-L-2,3-二氨丙酸结构式

|
常用名 | N(α)-Boc-L-2,3-二氨丙酸 | 英文名 | N(Alpha)-Boc-L-2,3-Diaminopropionic Acid |
|---|---|---|---|---|
| CAS号 | 73259-81-1 | 分子量 | 204.22 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 364.4±37.0 °C at 760 mmHg | |
| 分子式 | C8H16N2O4 | 熔点 | 210ºC (dec.) | |
| MSDS | 美版 | 闪点 | 174.2±26.5 °C |
|
Glucosamine-6-phosphate synthase from Escherichia coli: determination of the mechanism of inactivation by N3-fumaroyl-L-2,3-diaminopropionic derivatives.
Biochemistry 29 , 3668, (1990) A mechanistic investigation of the inactivation of Escherichia coli glucosamine-6-phosphate synthase by N3-(4-methoxyfumaroyl)-L-2,3-diaminopropionate (FMDP) was undertaken. On the basis of the known participation of the N-terminal cysteine residue in this pr... |
|
|
Antimicrobial properties of N3-(iodoacetyl)-L-2,3-diaminopropanoic acid-peptide conjugates.
J. Med. Chem. 33 , 2755, (1990) Six peptide conjugates consisting of either norvaline, methionine, or lysine and N3-(iodoacetyl)-L-2,3-diaminopropanoic acid--a strong, irreversible inactivator of bacterial and fungal glucosamine-6-phosphate synthase--were synthesized and their antibacterial... |
|
|
Minimum requirements for inhibition of smooth-muscle myosin light-chain kinase by synthetic peptides.
Biochem. J. 257 , 73, (1989) Although the amino acid residues that are important for peptide substrates of myosin light-chain kinase have been reported, those that are important for peptide inhibitors of this enzyme have not previously been investigated. Synthetic peptides based on the s... |
|
|
F. Ruan et al.
J. Org. Chem. 56 , 4347, (1991)
|