L-叔亮氨酸
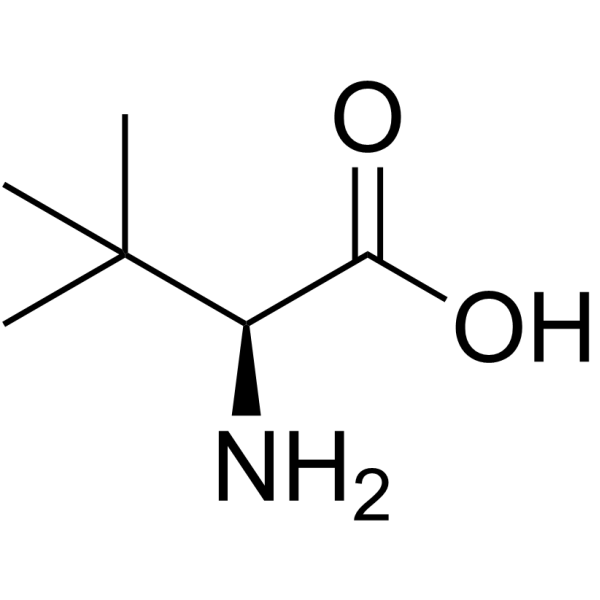
L-叔亮氨酸结构式

|
常用名 | L-叔亮氨酸 | 英文名 | L-tert-Leucine |
|---|---|---|---|---|
| CAS号 | 20859-02-3 | 分子量 | 131.173 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 217.7±23.0 °C at 760 mmHg | |
| 分子式 | C6H13NO2 | 熔点 | 300 °C | |
| MSDS | 中文版 美版 | 闪点 | 85.5±22.6 °C |
|
Highly selective asymmetric acetate aldol reactions of an N-acetyl thiazolidinethione reagent.
Org. Lett. 6 , 23-25, (2004) [reaction: see text] A highly diastereoselective acetate aldol reaction that uses a tert-leucine-derived thiazolidinethione auxiliary and dichlorophenylborane has been developed. The reaction proceeds in excellent yields and with high diastereoselectivities (... |
|
|
Scaleable catalytic asymmetric Strecker syntheses of unnatural alpha-amino acids.
Nature 461(7266) , 968-70, (2009) Alpha-amino acids are the building blocks of proteins and are widely used as components of medicinally active molecules and chiral catalysts. Efficient chemo-enzymatic methods for the synthesis of enantioenriched alpha-amino acids have been developed, but it ... |
|
|
An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pure D-tert-leucine.
Org. Lett. 5(20) , 3649-50, (2003) [reaction: see text] d-tert-Leucine was prepared with an enantiomeric excess of >99% by an enzyme-catalyzed oxidative resolution of the racemic mixture of dl-tert-leucine with use of leucine dehydrogenase. The l-amino acid was oxidized completely due to coupl... |
|
|
Effect of organic cosolvent on kinetic resolution of tert-leucine by penicillin G acylase from Kluyvera citrophila.
Bioprocess Biosyst. Eng. 28(5) , 285-9, (2006) Penicillin G acylase (PGA) from Kluyvera citrophila immobilized on Amberzyml was used for enantioselective hydrolysis of N-phenylacetylated-DL-tert-leucine (N-Phac-DL-Tle) to produce L-tert-leucine (L-Tle). The effects of various organic cosolvents on hydroly... |
|
|
Preferred conformations of peptides containing tert-leucine, a sterically demanding, lipophilic alpha-amino acid with a quaternary side-chain Cbeta atom.
Chemistry 11(8) , 2395-404, (2005) Terminally protected homopeptides of tert-leucine, from the dimer to the hexamer, co-oligopeptides of tert-leucine in combination with alpha-aminoisobutyric acid or glycine residues up to the hexamer level, and simple dipeptides representing known scaffolds f... |
|
|
The tert-butyl group in chemistry and biology.
Org. Biomol. Chem. 6(15) , 2655-65, (2008) The unique reactivity pattern elicited by the crowded tert-butyl group is highlighted by summarising characteristic applications. Starting from the use of this simple hydrocarbon moiety in chemical transformations, via its relevance in Nature and its implicat... |
|
|
Stabilizing and destabilizing effects of placing beta-branched amino acids in protein alpha-helices.
Biochemistry 33(40) , 12022-31, (1994) In order to gain greater insight into the effects of beta-branched amino acids on protein alpha-helices, hydrophobic amino acids with varying degrees of beta-branching, including the fully beta-substituted L-2-amino-3,3-dimethylbutanoic acid (ADBA), were inco... |
|
|
Enzyme-assisted preparation of D-tert.-leucine.
Biosci. Biotechnol. Biochem. 65(9) , 1977-80, (2001) Optically pure D-tert.-leucine was obtained by the enzymatic hydrolysis of (+/-)-N-acetyl-tert. leucine chloroethyl ester after about 50% conversion, this being catalyzed by a protease from Bacillus licheniformis (Alcalase), and subsequent acidic saponificati... |
|
|
Asymmetric construction of spirocyclohexanonerhodanines catalyzed by simple diamine derived from chiral tert-leucine.
Chem. Commun. (Camb.) 48(73) , 9180-2, (2012) A diamine-catalyzed asymmetric tandem reaction between α,β-unsaturated ketones and rhodanine derivatives has been developed to synthesize various spirocyclic compounds with high stereoselectivities (up to 99% ee and >20:1 dr). The products obtained contain tw... |