(S)-2-氨基-3-(5-氟-1H-吲哚-3-基)丙酸
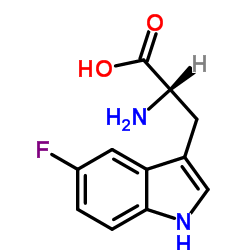
(S)-2-氨基-3-(5-氟-1H-吲哚-3-基)丙酸结构式

|
常用名 | (S)-2-氨基-3-(5-氟-1H-吲哚-3-基)丙酸 | 英文名 | DL-5-Fluorotryptophan |
|---|---|---|---|---|
| CAS号 | 16626-02-1 | 分子量 | 222.216 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | 450.7±45.0 °C at 760 mmHg | |
| 分子式 | C11H11FN2O2 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 226.4±28.7 °C |
|
Glucose recognition proteins for glucose sensing at physiological concentrations and temperatures.
ACS Chem. Biol. 9(7) , 1595-602, (2014) Advancements in biotechnology have allowed for the preparation of designer proteins with a wide spectrum of unprecedented chemical and physical properties. A variety of chemical and genetic methods can be employed to tailor the protein's properties, including... |
|
|
Substrate promiscuity of the cyclic dipeptide prenyltransferases from Aspergillus fumigatus ( section sign).
J. Nat. Prod. 72 , 44-52, (2009) This study reports that a series of tryptophan derivatives with modifications on the side chain or at the indole ring were accepted by two cyclic dipeptide prenyltransferases, CdpNPT and FtmPT1, and converted to prenylated derivatives. The structures of the e... |
|
|
Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin.
J. Med. Chem. 49(15) , 4616-22, (2006) Tryptophans at positions 4 and 7 of compstatin, a peptide complement inhibitor, are crucial for its interaction with C3. However, the nature of their involvement has not been studied to date. Here we investigate the molecular forces involved in the C3-compsta... |
|
|
Nuclear magnetic resonance and molecular genetic studies of the membrane-bound D-lactate dehydrogenase of Escherichia coli.
Biochemistry 26 , 549, (1987) In this study we demonstrate the potential of combining fluorine-19 nuclear magnetic resonance (NMR) spectroscopy with molecular genetics. We are using the membrane-bound enzyme D-lactate dehydrogenase of Escherichia coli as a model system to characterize int... |
|
|
Isomerization of (3S)-2,3-dihydro-5-fluoro-L-tryptophan and of 5-fluoro-L-tryptophan catalyzed by tryptophan synthase: studies using fluorine-19 nuclear magnetic resonance and difference spectroscopy.
Biochemistry 25 , 4240, (1986) We are exploring the active site and the mechanism of the pyridoxal phosphate dependent reactions of the bacterial tryptophan synthase alpha 2 beta 2 complex by use of substrate analogues and of reaction intermediate analogues. Fluorine-19 nuclear magnetic re... |
|
|
Ionization potentials of fluoroindoles and the origin of nonexponential tryptophan fluorescence decay in proteins.
J. Am. Chem. Soc. 127(11) , 4104-13, (2005) This work reports an explanation for the unusual monoexponential fluorescence decay of 5-fluorotryptophan (5FTrp) in single-Trp mutant proteins [Broos, J.; Maddalena, F.; Hesp, B. H. J. Am. Chem. Soc. 2004, 126, 22-23] and substantially clarifies the origin o... |
|
|
In vivo synthesized proteins with monoexponential fluorescence decay kinetics.
J. Am. Chem. Soc. 126(1) , 22-3, (2004) Tryptophan, when in a protein, typically shows multiexponential fluorescence decay kinetics. Complex kinetics prevents a straightforward interpretation of time-resolved fluorescence protein data, particularly in anisotropy studies or if the effect of a dynami... |
|
|
5-fluoro-D,L-tryptophan as a dual NMR and fluorescent probe of α-synuclein.
Methods Mol. Biol. 895 , 197-209, (2012) Analysis of conventional proton nuclear magnetic resonance (NMR) experiments on intrinsically disordered proteins (IDPs) is challenging because of the highly flexible and multiple rapidly exchanging conformations typifying this class of proteins. One method t... |
|
|
[5-fluoro-tryptophan-containing N-terminal domain of the alpha-subunit of the Torpedo californica acetylcholine receptor: preparation in E. coli and 19F NMR study].
Bioorg. Khim. 29(4) , 384-90, (2003) A protein corresponding to the extracellular 1-209 domain of the alpha-subunit of the nicotine acetylcholine receptor from the electric organ of Torpedo californica was prepared using the corresponding cDNA domain by culturing Escherichia coli cells on a synt... |
|
|
Determination of the 19F NMR chemical shielding tensor and crystal structure of 5-fluoro-dl-tryptophan.
J. Magn. Reson. 187(1) , 88-96, (2007) 5-Fluoro-dl-tryptophan (5F-Trp) is a very sensitive probe used to investigate orientation and dynamics of biomacromolecules at the in situ level. In order to establish a (19)F NMR strategy, the crystal structure and (19)F chemical shielding tensor of 5F-Trp a... |