那格列奈相关物质C
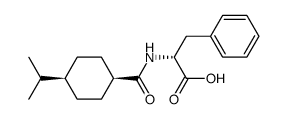
那格列奈相关物质C结构式

|
常用名 | 那格列奈相关物质C | 英文名 | D-Phenylalanine, N-[[4-(1-methylethyl)cyclohexyl]carbonyl]-, cis |
|---|---|---|---|---|
| CAS号 | 105816-06-6 | 分子量 | 317.42300 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C19H27NO3 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | N/A |
|
Formulation and in vitro evaluation of nateglinide microspheres using HPMC and carbopol-940 polymers by ionic gelation method.
Pak. J. Pharm. Sci. 26(6) , 1229-35, (2013) This study involves the design and characterization of Nateglinide (NAT) microspheres to enhance patient compliance. Ionic gelation technique was used to prepare Nateglinide Microspheres by using rate controlling polymers Carbopol-940 and Hydroxypropylmethyl ... |
|
|
II. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: co-milling.
Int. J. Pharm. 454(1) , 568-72, (2013) Nateglinide is an oral antidiabetic agent that should be administered 10-30 min before the meal, but it shows low and pH-dependent solubility that may reduce its oral bioavailability. To improve nateglinide dissolution rate, the active was co-milled with thre... |
|
|
I. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: nanoparticles and co-mixing.
Int. J. Pharm. 454(1) , 562-7, (2013) Nateglinide is a non-sulphonylurea insulinotropic oral antidiabetic agent. The main problem in formulating an oral dosage form is its low solubility in aqueous media. This problem is particularly critical for an anti-diabetic drug because it should be adminis... |
|
|
Estimating relative stability of polymorphs by generation of configurational free energy phase diagram.
J. Pharm. Sci. 101(5) , 1843-51, (2012) Relationship between two polymorphs is described to be either enantiotropic or monotropic with transition temperature/transition point (T(t) ) below the melting point (T(m) ) of the lower melting form in former case and above the T(m) of the higher melting fo... |
|
|
Synthesis and characterization of carboxymethyl chitosan hydrogel: application as pH-sensitive delivery for nateglinide.
Curr. Drug Deliv. 9(6) , 628-36, (2012) In current research, chitosan was reacted with mono-chloroacetic acid under alkaline condition to prepare carboxymethyl chitosan (CMCTs). The degree of substitution (Ds) on prepared CMCTs was found to be 0.68. CMCTs was used as a potential carrier for pH spec... |
|
|
Relative fasting bioavailability of two formulations of nateglinide 60 mg in healthy male Chinese volunteers: an open-label, randomized-sequence, single-dose, two-way crossover study.
Clin. Ther. 34(7) , 1505-10, (2012) Nateglinide, N-(trans-4-isopropylcyclohexyl-carbonyl)-d-phenylalanine, is a potent insulin secretagogue designed to restore early-phase insulin secretion. It increases pancreatic insulin secretion by competitively binding to sulfonylurea receptors inhibiting ... |
|
|
Contribution of organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 to hepatic uptake of nateglinide, and the prediction of drug-drug interactions via these transporters.
J. Pharm. Pharmacol. 64(2) , 199-206, (2012) We have investigated the contributions of organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 to the hepatic uptake of nateglinide, and the possibility of drug-drug interactions via these transporters.Uptake studies using transporter-expressing HEK2... |
|
|
Incidence of atrial fibrillation in a population with impaired glucose tolerance: The contribution of glucose metabolism and other risk factors. A post hoc analysis of the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial
Am. Heart J. 166(5) , 935-40.e1, (2013) Background The role of dysglycemia as an additional risk factor for atrial fibrillation (AF) is controversial. Therefore, it was of interest to assess risk factors for incident AF in a large, representative population of patients with cardiovascular risk fact... |
|
|
Effect of CYP2C9 and SLCO1B1 polymorphisms on the pharmacokinetics and pharmacodynamics of nateglinide in healthy Chinese male volunteers.
Eur. J. Clin. Pharmacol. 69(3) , 407-13, (2013) Nateglinide is commonly used in the treatment of patients with type 2 diabetes mellitus. Our objective was to assess the association between CYP2C9 and SLCO1B1 polymorphisms and the metabolism of nateglinide in healthy Chinese male volunteers.A total of 35 he... |
|
|
Nateglinide in combination with metformin in Chinese patients with type 2 diabetes mellitus: a post-marketing surveillance study.
Clin. Drug Investig. 33(3) , 185-91, (2013) Diabetes mellitus has become a major public health problem in China. This open-label, prospective, multicentre, post-marketing surveillance study was conducted to investigate the efficacy and safety of nateglinide in combination with metformin in Chinese pati... |