2,4-戊二醇
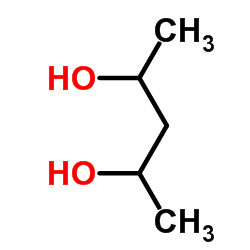
2,4-戊二醇结构式

|
常用名 | 2,4-戊二醇 | 英文名 | 2,4-PENTANEDIOL |
|---|---|---|---|---|
| CAS号 | 625-69-4 | 分子量 | 104.148 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 199.5±0.0 °C at 760 mmHg | |
| 分子式 | C5H12O2 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 101.7±0.0 °C |
|
Brønsted acid catalyzed asymmetric reduction of ketones and acyl silanes using chiral anti-pentane-2,4-diol.
Org. Lett. 12(10) , 2294-7, (2010) Ketones and acyl silanes were reduced to the corresponding alcohols by a simple procedure employing anti-1,3-diol and a catalytic amount (5 mol %) of 2,4-dinitrobenzenesulfonic acid in benzene at reflux. Asymmetric induction reached up to >99% ee when a chira... |
|
|
Highly efficient synthesis of enantiopure diacetylated C(2)-symmetric diols by ruthenium- and enzyme-catalyzed dynamic kinetic asymmetric transformation (DYKAT).
Chemistry 12 , 6053, (2006) Highly efficient synthesis of enantiopure diacetates of 2,4-pentanediol and 2,5-hexanediol starting from commercially available mixtures of the diols (dl/meso approximately 1:1) has been realized by combining a fast ruthenium-catalyzed epimerization with an e... |
|
|
2,4-Pentanediolate as an alkoxide/diketonate "hybrid" ligand and the formation of aluminum and zirconium derivatives.
Inorg. Chem. 50(23) , 12126-32, (2011) When 2,4-pentanediol (2,4-H(2)pd) is deprotonated, the resulting dianion (2,4-pd) serves as a type of "hybrid" ligand, i.e., an alkoxide that possesses structural features of a β-diketonate. 2,4-Pentanediol reacts with Al(O-s-Bu)(3) and Zr(O-i-Pr)(4) to form ... |
|
|
Theoretical insight into stereoselective reaction mechanisms of 2,4-pentanediol-tethered ketene-olefin [2 + 2] cycloaddition.
J. Phys. Chem. A 116(4) , 1168-75, (2012) We report ab initio molecular dynamics calculations based on density functional theory performed on an intramolecular [2 + 2] cycloaddition between ketene and olefin linked with a 2,4-pentanediol (PD) tether. We find that the encounter of the ketene and olefi... |
|
|
Lack of selectivity between anesthetic stereoisomers for an inhibitory site which is located on a membrane protein.
Biochim. Biophys. Acta 1026(1) , 40-2, (1990) Lack of selectivity towards anesthetic stereoisomers is one of the few criteria available for the identification of an anesthetic target site. Until now this criterion has not been tested for anesthetics that directly interact with sensitive membrane proteins... |