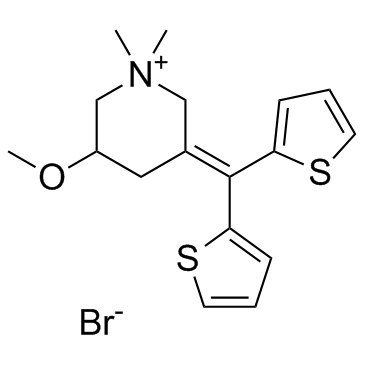
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TN5083000
-
CHEMICAL NAME :
-
Piperidinium, 3-(di-2-thienylmethylene)-5-methoxy-1,1-dimethyl-, bromide
-
CAS REGISTRY NUMBER :
-
35035-05-3
-
LAST UPDATED :
-
199209
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C17-H22-N-O-S2.Br
-
MOLECULAR WEIGHT :
-
400.43
-
WISWESSER LINE NOTATION :
-
T6K CYTJ A1 A1 EO1 CUY- BT5SJ&- BT5SJ &E
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1213 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,685,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
55 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,685,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
254 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,685,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
7 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Autonomic Nervous System - smooth muscle relaxant (mechanism undefined, spasmolytic)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 23,461,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
324 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 17,2087,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
713 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - other changes
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 15,914,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
97 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - other changes
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 15,914,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
145 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,685,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JJPAAZ Japanese Journal of Pharmacology. (Japanese Pharmacological Soc., c/o Dept. of Pharmacology, Faculty of Medicine, Kyoto Univ., Sakyo-ku, Kyoto 606, Japan) V.1- 1951- Volume(issue)/page/year: 22,685,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
199 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,351,1982 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
17500 mg/kg/35D-C
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Gastrointestinal - hypermotility, diarrhea Liver - hepatitis (hepatocellular necrosis), zonal
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 23,9,1982 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
960 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 7,1293,1973
|