71376-34-6
| 中文名 | 串珠镰刀菌素 |
|---|---|
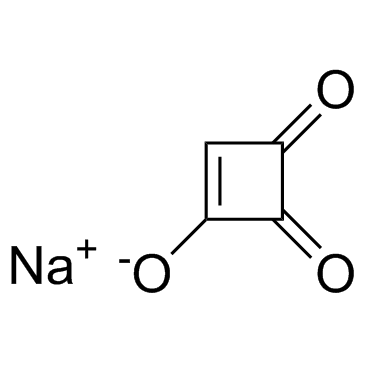
| 英文名 | sodium,3,4-dioxocyclobuten-1-olate |
| 英文别名 |
3-CYCLOBUTENE-1,2-DIONE,3-HYDROXY-,SODIUM SALT
1-Hydroxycyclobut-1-ene-3,4-dione Moniliformin sodium salt from Fusarium proliferatum 3-Hydroxy-3-cyclobutenedione sodium salt Moniliformin sodium salt 1-Hydroxycyclobut-1-ene-3,4-dione sodium salt Moniliformin (sodium salt) |
| 描述 | Moniliformin sodium salt是提取自镰孢镰刀菌中的有效,水溶性的霉菌毒素。 |
|---|---|
| 相关类别 | |
| 体外研究 | Fusarium moniliforme NRRL 6322每kg玉米砂粒培养基产生约600mg可回收的moniliformin,一种霉菌毒性代谢物。几种Fusarium moniliforme菌株在实验室培养中每千克生长基质产生超过800毫克的moniliformin [1]。在分化过程中暴露于moniliformin的单核细胞衍生的巨噬细胞表现出内吞作用能力的降低,以及CD71和HLA-DR表达的降低[2]。 |
| 体内研究 | Moniliformin对小鼠的毒性较小,女性的LD50为每公斤20.9毫克,男性为每公斤体重29.1毫克。与小鸡的情况一样,在毒素中存活的小鼠没有表现出任何不良影响;死后的小鼠或小鸡在治疗后4至6小时内卧位,并在24小时内死亡。在4日龄鸡胚中,获得了每个胚胎2.8微克的尖锐LD50,幸存者没有明显的致畸作用[1]。用最高剂量的念尼泊定治疗的大鼠显示活性降低,然后是急性心力衰竭和死亡。较低剂量(<9mg/kg体重)的大鼠未显示出毒性迹象。每日摄入的moniliformin强烈降低了所有剂量组中嗜中性粒细胞的吞噬活性。在随访期间,卫星组继续减少,表明对免疫系统有严重影响,而对于念珠菌素,LOAEL值为3mg/kg体重。 Moniliformin迅速排泄到尿液中,每日摄入量在20.2%至31.5%之间,并且没有积累的迹象。粪便中moniliformin的浓度小于2%,这表明从胃肠道有效吸收[3]。 |
| 动物实验 | 大鼠:Moniliformin在水中制备。在该实验中,将5个剂量组(3,6,9,12和15mg / kg moniliformin bw)的试验动物暴露于moniliformin 28天。每组由5只雄性Sprague-Dawley大鼠组成。剂量组基于我们对大鼠MON的急性毒性研究确定。另外,使用给予过滤自来水和两个卫星组(给药12和15mg / kg体重的moniliformin)的对照组。两个卫星组在没有治疗的情况下再保持14天,以检测可能的延迟毒性作用并跟踪恢复情况[3]。小鼠:将念珠菌素的无菌水溶液腹膜内(0.2mL)注射到5只雌性和5只雄性白色小鼠中,每只体重约25g,浓度相当于每kg体重0,20,25,30和35mg。在4天的时间内观察小鼠,并测定LD 50值[1]。 |
| 参考文献 |
| 沸点 | 239.6ºC at 760 mmHg |
|---|---|
| 分子式 | C4HNaO3 |
| 分子量 | 120.03900 |
| 闪点 | 113ºC |
| 精确质量 | 119.98200 |
| PSA | 57.20000 |
| 储存条件 | Store at 2-8 |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Moniliformin sodium salt, from Fusarium proliferatum REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later
registration deadline. CAS-No.: 71376-34-6 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Acute toxicity, Oral (Category 3), H301 For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/EC T ToxicR25 For the full text of the R-phrases mentioned in this Section, see Section 16. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal wordDanger Hazard statement(s) H301Toxic if swallowed. Precautionary statement(s) P301 + P310IF SWALLOWED: Immediately call a POISON CENTER or doctor/ physician. Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Chemical characterization : Natural product Synonyms: 1-Hydroxycyclobut-1-ene-3,4-dione Formula: C4HNaO3 Molecular Weight: 120,04 g/mol CAS-No.: 71376-34-6 Hazardous ingredients according to Regulation (EC) No 1272/2008 ComponentClassificationConcentration Moniliformin CAS-No.71376-34-6Acute Tox. 3; H301<= 100 % Hazardous ingredients according to Directive 1999/45/EC ComponentClassificationConcentration Moniliformin CAS-No.71376-34-6T, R25<= 100 % For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, Sodium oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8. Environmental precautions Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2 - 8 °C Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling the product. Personal protective equipment Eye/face protection Face shield and safety glasses Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Full contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) Splash contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an industrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario. Body Protection Complete suit protecting against chemicals, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Where risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Prevent further leakage or spillage if safe to do so. Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity LD50 Intraperitoneal - mouse - 21 mg/kg Remarks: Behavioral:Altered sleep time (including change in righting reflex). Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: GU1815000 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: 2811IMDG: 2811IATA: 2811 UN proper shipping name ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (Moniliformin) IMDG: TOXIC SOLID, ORGANIC, N.O.S. (Moniliformin) IATA:Toxic solid, organic, n.o.s. (Moniliformin) Transport hazard class(es) ADR/RID: 6.1IMDG: 6.1IATA: 6.1 Packaging group ADR/RID: IIIMDG: IIIATA: II Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |

GHS06 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H301 |
| 警示性声明 | P301 + P310 |
| 个人防护装备 | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| 危害码 (欧洲) | T: Toxic; |
| 风险声明 (欧洲) | 25 |
| 安全声明 (欧洲) | 45 |
| 危险品运输编码 | UN 2811 6.1/PG 2 |
| RTECS号 | GU1815000 |