42017-89-0
| 中文名 | 非诺贝特酸 |
|---|---|
| 英文名 | fenofibric acid |
| 中文别名 |
2-(4-(4-氯苯甲酰)苯氧基)-2-甲基丙酸
非诺贝酸 2-[4-(4-氯苯甲酰基)苯氧基]-2-甲基丙酸 |
| 英文别名 |
Procetofenic acid
2-(4'-(p-chlorobenzoyl)phenoxy)-2-methylpropionic acid 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methyl-propanoic acid Fibricor Fenofibric acid Fenofibrate Related Compound B 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid Fenofibrate impurity B EINECS 255-626-9 MFCD00792461 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropanoic acid |
| 描述 | Fenofibric acid 是 fenofibrate 的活性代谢物,为 PPAR 激动剂,对 PPARα,PPARγ 和 PPARδ 的 EC50 值分别为 22.4 µM,1.47 µM 和 1.06 µM;Fenofibric acid 同时可抑制 COX-2 的活性,IC50 值为 48 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
PPARδ:1.06 μM (EC50) PPARγ:1.47 μM (EC50) PPARα:22.4 μM (EC50) COX-2:48 μM (IC50) |
| 体外研究 | Fenofibric acid是一种PPAR激活剂,PPARα,PPARγ和PPARδ的EC50分别为22.4μM,1.47μM和1.06μM[1]。非诺贝酸(10,25,50,75和100 nM)剂量依赖性地抑制COX-2酶,IC50为48 nM [2]。非诺贝酸(500 nM)可降低HepG2细胞中AOX1蛋白的丰度[3]。 Fenofibric acid(100μM)可降低JNK1/2,c-Jun和p38 MAPK的磷酸化水平,并可防止活性氧,内质网(ER)应激和血液视网膜屏障(BRB)破坏的积累。 ARPE-19细胞中的高葡萄糖(HG)和缺氧。在HG条件和缺氧条件下,非诺贝酸(100μM)激活ARPE-19细胞中IGF-IR/Akt/ERK1/2介导的存活信号通路[4]。 |
| 体内研究 | Fenofibric acid(1,5,10 mg/kg,po)在由角叉菜胶诱导的急性炎症的Wistar大鼠中显示出抗炎活性[2]。 |
| 细胞实验 | ARPE-19细胞在正常血糖(5.5mM D-葡萄糖)或高血糖(25mM D-葡萄糖)条件下在37℃,5%(v / v)CO 2下培养18天,培养基DMEM / F12补充10% (v / v)胎儿血清(FS)和青霉素/链霉素。使用ARPE-19细胞,每3-4天更换培养基。测试的条件是:(1)将对照细胞维持在5.5mM D-葡萄糖(正常葡萄糖)中18天。 (2)在用100μM非诺贝酸处理的5.5mM D-葡萄糖中培养72小时(第16,17和18天;每天施用1次)的细胞。 (3)如(1)或(2)中培养的细胞,并在最后6或24小时内进行缺氧(1%氧气)。 (4)细胞维持在25mM D-葡萄糖(HG)中18天。 (5)在用100μM非诺贝酸处理的25mM D-葡萄糖中培养72小时(第16,17和18天;每天施用1次)的细胞。 (6)如(4)或(5)中培养的细胞,在最后6或24小时内进行缺氧(1%氧气)[4]。 |
| 动物实验 | 通过将0.1mL在盐水(足底下)中制备的1%角叉菜胶溶液注射到大鼠的右后爪来评估非诺贝特及其活性代谢物fenofibric acid的抗炎活性。将大鼠分成6组,每组6只动物。第一组用作阴性对照,并在蒸馏水中接受1%吐温-80,10mL / kg体重。第2组和第3组接受单剂量非诺贝特和标准药物双氯芬酸10 mg / kg体重,而第4,5和6组分别接受3剂量的Fenofibric acid,分别为1,5和10 mg / kg体重。在通过足底下途径注射0.1mL的1%角叉菜胶之前60分钟使用管饲口服给予所有药物。在诱导炎症后0,1,2和3小时使用体积描记器测量测试组和对照组的水肿体积[2]。 |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 486.5±35.0 °C at 760 mmHg |
| 熔点 | 176--179ºC |
| 分子式 | C17H15ClO4 |
| 分子量 | 318.752 |
| 闪点 | 248.0±25.9 °C |
| 精确质量 | 318.065887 |
| PSA | 63.60000 |
| LogP | 3.86 |
| 外观性状 | 白色或近乎于白色结晶粉末 |
| 蒸汽压 | 0.0±1.3 mmHg at 25°C |
| 折射率 | 1.585 |
| 储存条件 | -20°C Freezer |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:1 3.氢键受体数量:4 4.可旋转化学键数量:5 5.互变异构体数量:无 6.拓扑分子极性表面积:63.6 7.重原子数量:22 8.表面电荷:0 9.复杂度:405 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Fenofibric acid REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 42017-89-0 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Acute toxicity, Oral (Category 4), H302 Acute aquatic toxicity (Category 1), H400 Chronic aquatic toxicity (Category 1), H410 For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/EC Xn, N Harmful, Dangerous for the R22, R50/53 environment For the full text of the R-phrases mentioned in this Section, see Section 16. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal wordWarning Hazard statement(s) H302Harmful if swallowed. H410Very toxic to aquatic life with long lasting effects. Precautionary statement(s) P273Avoid release to the environment. P501Dispose of contents/ container to an approved waste disposal plant. Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Formula: C17H15ClO4 Molecular Weight: 318,75 g/mol CAS-No.: 42017-89-0 EC-No.: 255-626-9 Hazardous ingredients according to Regulation (EC) No 1272/2008 ComponentClassificationConcentration 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid CAS-No.42017-89-0Acute Tox. 4; Aquatic Acute 1;<= 100 % EC-No.255-626-9Aquatic Chronic 1; H302, H410 Hazardous ingredients according to Directive 1999/45/EC ComponentClassificationConcentration 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid CAS-No.42017-89-0Xn, N, R22 - R50/53<= 100 % EC-No.255-626-9 For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, Hydrogen chloride gas Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Avoid breathing dust. For personal protection see section 8. Environmental precautions Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Specific end use(s) Apart from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection Complete suit protecting against chemicals, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: powder Colour: white b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- log Pow: 3,895 octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity LD50 Oral - rat - 1.242 mg/kg Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: UA2453000 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects Very toxic to aquatic life with long lasting effects. SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: 3077IMDG: 3077IATA: 3077 UN proper shipping name ADR/RID: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (2-[4-(4- Chlorobenzoyl)phenoxy]-2-methylpropionic acid) IMDG: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (2-[4-(4- Chlorobenzoyl)phenoxy]-2-methylpropionic acid) IATA:Environmentally hazardous substance, solid, n.o.s. (2-[4-(4-Chlorobenzoyl)phenoxy]-2- methylpropionic acid) Transport hazard class(es) ADR/RID: 9IMDG: 9IATA: 9 Packaging group ADR/RID: IIIIMDG: IIIIATA: III Environmental hazards ADR/RID: yesIMDG Marine pollutant: yesIATA: yes Special precautions for user Further information EHS-Mark required (ADR 2.2.9.1.10, IMDG code 2.10.3) for single packagings and combination packagings containing inner packagings with Dangerous Goods > 5L for liquids or > 5kg for solids. SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |


GHS07, GHS09 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302-H410 |
| 警示性声明 | P273-P501 |
| 危害码 (欧洲) | Xn,N |
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | 26-36/37/39 |
| 危险品运输编码 | UN 3077 9 / PGIII |
| RTECS号 | UA2453000 |
| 海关编码 | 2918990090 |
|
~93% 
42017-89-0 |
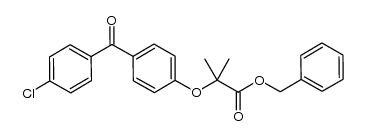
| 文献:AMPLA PHARMACEUTICALS INC. Patent: WO2009/73138 A2, 2009 ; Location in patent: Page/Page column 81-82 ; WO 2009/073138 A2 |
|
~72% 
42017-89-0 |
| 文献:SIGNATURE PHARMACEUTICALS, LLC Patent: WO2005/46575 A2, 2005 ; Location in patent: Page/Page column 176-177 ; |
|
~86% 
42017-89-0 |
| 文献:Davis, Roman D.; Fitzgerald, Russ N.; Guo, Jiasheng Synthesis, 2004 , # 12 p. 1959 - 1962 |
|
~98% 
42017-89-0 |
| 文献:Mattsson, Sara; Dahlstroem, Mikael; Karlsson, Staffan Tetrahedron Letters, 2007 , vol. 48, # 14 p. 2497 - 2499 |
|
~% 
42017-89-0 |
| 文献:Labor. Fournier Patent: FR2300552DE2605382 , 19761976 ; Chem.Abstr., 1976 , vol. 85, # 192382 |
|
~% 
42017-89-0 |
| 文献:Labor. Fournier Patent: FR2300552DE2605382 , 19761976 ; Chem.Abstr., 1976 , vol. 85, # 192382 |
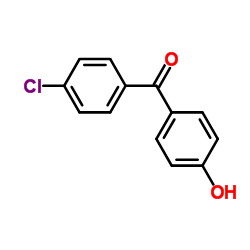
| 上游产品 8 | |
|---|---|
| 下游产品 4 | |
| 海关编码 | 2918990090 |
|---|---|
| 中文概述 | 2918990090. 其他含其他附加含氧基羧酸(包括酸酐、酰卤化物、过氧化物和过氧酸及该税号的衍生物). 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |




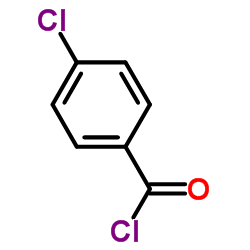

![propan-2-yl 2-[4-[(4-chlorophenyl)-hydroxymethyl]phenoxy]-2-methylpropanoate结构式](https://image.chemsrc.com/caspic/398/61001-99-8.png)
![2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoyl chloride结构式](https://image.chemsrc.com/caspic/055/65178-90-7.png)