7647-15-6
| 中文名 | 溴化钠 |
|---|---|
| 英文名 | sodium bromide |
| 中文别名 | 钠臭 |
| 英文别名 |
sodium boron
NaBr Sedoneural Natriumbromid SODIUM BROMID EINECS 231-599-9 Sodum bromide bromuredesodium bromnatrium sodiium bromide sodium bromine Sodiumbomide MFCD00003475 |
| 密度 | 3,203 g/cm3 |
|---|---|
| 沸点 | 1390 °C |
| 熔点 | 755 °C(lit.) |
| 分子式 | BrNa |
| 分子量 | 102.894 |
| 闪点 | 1390°C |
| 精确质量 | 101.908096 |
| 外观性状 | 白色粉末 |
| 蒸汽压 | 1 mm Hg ( 806 °C) |
| 折射率 | 1.6412 |
| 储存条件 | 1.应贮存在干燥、阴凉、通风良好的地方。要防止日光曝晒,与火种和热源隔离,不得与氨、氧、磷、锑粉和碱类共贮混运。应远离木屑、刨花、稻草等,以防燃烧。 2.失火时,可用沙土、二氧化碳灭火器扑救。 |
| 稳定性 | 1.在空气中有吸湿性。易溶于水(100 ℃时溶解度为121 g/100 ml水),水溶液呈中性。微溶于醇。51 ℃时溶液中析出无水溴化钠结晶,低于51 ℃则生成二水物。其溴离子可被氟、氯所取代。在酸性条件下,能被氧氧化,游离出溴。可与稀硫酸反应生成溴化氢。 |
| 水溶解性 | 905 g/L (20 ºC) |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:1 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积0 7.重原子数量:2 8.表面电荷:0 9.复杂度:2 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:2 |
| 更多 | 1.性状:无色立方晶系晶体或白色颗粒状粉末。无臭,味咸而微苦。 2.密度(g/mL,25 ℃ ): 3.203
3.熔点(ºC):755 4.沸点(ºC,常压):1390℃ 5.折射率:1.6412 6.闪点(ºC):1390 7溶解性:易溶于水(100℃时溶解度为121g/100ml水),水溶液呈中性。微溶于醇。51℃时溶液中析出无水溴化钠结晶,低于51℃则生成二水物。905g/L(20ºC时) |
Synonym:Bromide salt of sodium, Sedoneura Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Hygroscopic. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Effects may be cumulative. May cause central nervous system effects such as psychosis, hallucinations, depression, visual changes and mental changes. Ingestion may cause skin eruptions and rash. Inhalation: May cause effects similar to those described for ingestion. Chronic: Chronic inhalation and ingestion may cause effects similar to those of acute inhalation and ingestion. May cause incoordination and mental disturbances. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Skin: Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Treatment includes hydration, mild diuresis, and possible hemodialysis. Consider the use of ammonium chloride in divided doses with a diuretic. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Substance is noncombustible. Extinguishing Media: Use extinguishing media most appropriate for the surrounding fire. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with skin and eyes. Storage: Store in a cool, dry place. Store in a tightly closed container. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels. Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear a chemical apron. Respirators: Follow the OSHA respirator regulations found in 29CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Appearance: white Odor: Odorless pH: 6.5-8.0 Vapor Pressure: 1 mm Hg @ 806 deg C Viscosity: Not available. Boiling Point: 1390 deg C @ 760.00mm Hg Freezing/Melting Point: 755 deg C Autoignition Temperature: Not available. Flash Point: 800 deg C ( 1,472.00 deg F) Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: 800 deg C Solubility in water: 95g/100ml water (25 c) Specific Gravity/Density: 3.21g/cm3 Molecular Formula: BrNa Molecular Weight: 102.8938 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, heating to decomposition, exposure to moist air or water. Incompatibilities with Other Materials: Strong acids - alkaloidal and heavy metal salts (lead, silver, manganese, antimony, mercury, etc.) - strong oxidizing agents - halogen halides - bromine trifluoride. Hazardous Decomposition Products: Hydrogen bromide, sodium oxide, bromine fumes. Hazardous Polymerization: Has not been reported Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 7647-15-6: VZ3150000 LD50/LC50: CAS# 7647-15-6: Oral, mouse: LD50 = 7 gm/kg; Oral, rat: LD50 = 3500 mg/kg. Carcinogenicity: Sodiumbromide - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Ecotoxicity: Water danger/protection: WGK 1 Bluegill sunfish LC50: >1000 mg/l/96H Rainbow trout LC50: >1000 mg/l/96H Daphnia magna LC50: >1000 mg/l/48H Bobwhite quail LD50: >2250 mg/kg Mallard duck LC50: >5633 ppm Bobwhite quail LC50: >5633 ppm Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Shipping Name: Not regulated. Dangerous Goods Code: UN Number: Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 7647-15-6: 1 United Kingdom Occupational Exposure Limits Canada CAS# 7647-15-6 is listed on Canada's DSL List. CAS# 7647-15-6 is listed on Canada's Ingredient Disclosure List. Exposure Limits US FEDERAL TSCA CAS# 7647-15-6 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 急性毒性:LD50:7000mg/Kg(大鼠经口);3500mg/Kg(兔经皮) 低毒,半数致死量(大鼠,经口)3500mg/kg。 要防止摄入、吸入,防止眼睛、皮肤与之接触。
生态学数据: 水危害级别1:对水是稍微危害的,不要让未稀释或大量的产品接触地下水,水道或者污水系统。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xi: Irritant; |
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | S24/25-S25 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 1 |
| RTECS号 | VZ3150000 |
| 海关编码 | 2827510000 |
| 上游产品 7 | |
|---|---|
| 下游产品 8 | |
| 海关编码 | 2827510000 |
|---|


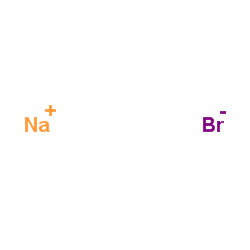







 加热赶出二氧化碳气体并浓缩溶液至结晶,然后冷却至室温,析出的结晶用少量水溶解,并滴加溴水至刚出现溴的颜色,加入硫化氢溶液进行脱色,加热蒸发浓缩结晶至干,高温析出无水溴化物。110℃下保温干燥后在保干器中冷却,即得无水溴化物。
加热赶出二氧化碳气体并浓缩溶液至结晶,然后冷却至室温,析出的结晶用少量水溶解,并滴加溴水至刚出现溴的颜色,加入硫化氢溶液进行脱色,加热蒸发浓缩结晶至干,高温析出无水溴化物。110℃下保温干燥后在保干器中冷却,即得无水溴化物。