52212-02-9
| 中文名 | 哌库溴铵 |
|---|---|
| 英文名 | 4,4'-((2beta,3alpha,5alpha,16beta,17beta)-3,17-Bis(acetyloxy)androstane-2,16-diyl)bis(1,1-dimethyl-piperazinium) dibromide |
| 中文别名 |
4,4'-[(2Β,3Α,5Α,16Β,17Β)-3,17-双(乙酰氧基)雄甾烷-2,16-亚基]双(1,1-二甲基哌嗪锚)二溴化物
阿端 |
| 英文别名 |
PIPECURINIUM BROMIDE
PIPECURIUM BROMIDE Ardtm Pipecuronium RGH-1106 EINECS 257-740-4 Arduan (tn) pipecuronium bromide Pipecuroniumbromid Pipecuriurn Brmnide Arduan |
| 描述 | Pipecuronium bromide 是一种有效的长效非去极化甾体神经肌肉阻滞剂 (NMBA),是一种双季铵化合物。Pipecuronium bromide 是一种功能强大的竞争性 nAChR 拮抗剂,Kd 为 3.06 μM。 |
|---|---|
| 相关类别 | |
| 靶点 |
nAChR[4][5] |
| 体外研究 | 糖胺对溴化哌库宁有很高的亲和力。由于哌库溴铵的效力是罗库溴铵的6至7倍,因此实现相对阻断所需的分子比罗库溴铵少[1]。 |
| 体内研究 | 平均ED95为0.045mg/kg(0.035-0.059mg/kg),起效时间为2-6.3min,取决于剂量和背景麻醉。哌库溴铵不释放组胺,即使在3×ED95剂量下也无心血管副作用,过敏反应似乎不存在问题〔2〕。在离体大鼠膈肌实验中,羧甲基化γ-环糊精显示了哌库溴铵诱导的神经肌肉阻滞的有效和完全逆转[3]。 |
| 参考文献 |
| 密度 | 1.329 |
|---|---|
| 熔点 | 262-264ºC |
| 分子式 | C35H62Br2N4O4 |
| 分子量 | 762.69900 |
| 精确质量 | 760.31400 |
| PSA | 59.08000 |
| 储存条件 | 2-8°C, 密封, 干燥 |
| 更多 | 1.性状:从二氯甲烷-丙酮结晶。 2.熔点:262-264℃(分解)。 3.比旋光度:[α] D23+8.1°(C=1,水)。 |
|
毒理学数据: 急性毒性LD50小鼠,大鼠(mg/kg):29.7,172.6静脉注射;70.6,449.6腹腔注射。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危害码 (欧洲) | Xi |
|---|---|
| 风险声明 (欧洲) | R36/37/38:Irritating to eyes, respiratory system and skin . |
| 安全声明 (欧洲) | S26-S36 |
| WGK德国 | 3 |
| RTECS号 | GN6820000 |
| 海关编码 | 29322980 |
|
~92% 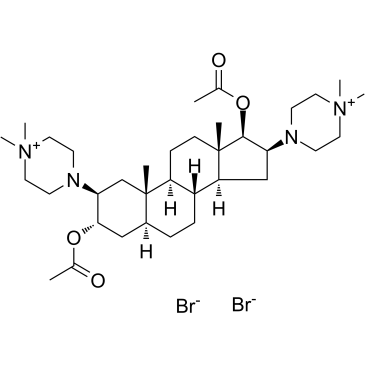
52212-02-9 |
| 文献:Tuba Arzneimittel-Forschung/Drug Research, 1980 , vol. 30, # 2 A p. 342 - 346 |
| 上游产品 1 | |
|---|---|
| 下游产品 0 | |
| 海关编码 | 29322980 |
|---|