4-Ethylbenzonitrile
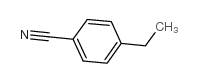
4-Ethylbenzonitrile structure
|
Common Name | 4-Ethylbenzonitrile | ||
|---|---|---|---|---|
| CAS Number | 25309-65-3 | Molecular Weight | 131.17400 | |
| Density | 0.956 g/mL at 25 °C(lit.) | Boiling Point | 237 °C(lit.) | |
| Molecular Formula | C9H9N | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 208 °F | |
|
Rational design of novel CYP2A6 inhibitors.
Bioorg. Med. Chem. 22(23) , 6655-64, (2015) Inhibition of CYP2A6-mediated nicotine metabolism can reduce cigarette smoking. We sought potent and selective CYP2A6 inhibitors to be used as leads for drugs useful in smoking reduction therapy, by evaluating CYP2A6 inhibitory effect of novel formyl, alkyl a... |
|
|
One-pot synthesis of 1,3,5-triazine derivatives via controlled cross-cyclotrimerization of nitriles: a mechanism approach.
J. Org. Chem. 79(15) , 7012-24, (2014) The reaction of equimolecular amounts of a nitrile and triflic anhydride or triflic acid at low temperature produces an intermediate nitrilium salt that subsequently reacts with 2 equiv of a different nitrile at higher temperature to form 2,4-disusbstituted-6... |
|
|
Visible light-promoted metal-free C-H activation: diarylketone-catalyzed selective benzylic mono- and difluorination.
J. Am. Chem. Soc. 135(46) , 17494-500, (2013) We report herein an operationally simple method for the direct conversion of benzylic C-H groups to C-F. We show that visible light can activate diarylketones to abstract a benzylic hydrogen atom selectively. Adding a fluorine radical donor yields the benzyli... |
|
|
Alkylphenols and arylnitriles in a biologically active neutral subfraction of cigarette smoke condensate. Miller RL and Stedman RL.
Phytochemistry 10(5) , 1135-1140, (1971)
|
|
|
1-Cyano-1, 3-butadienes. II. Carbon Structure of the Adduct Formed by the Diels--Alder Condensation of 1-Cyano-1, 3-butadiene with 1, 3-Butadiene. Snyder HR and Poos GI.
J. Am. Chem. Soc. 71(3) , 1057-1058, (1949)
|
|
|
Arrhenius parameters for rearrangements of the neophyl, 1-indanylmethyl, 2-allylbenzyl, and 2-(2-vinylphenyl) ethyl radicals relative to hydrogen abstraction from tributylstannane. Franz JA, et al.
J. Am. Chem. Soc. 106(14) , 3964-3967, (1984)
|
|
|
Yaws CL.
The Yaws Handbook of Vapor Pressure: Antoine coefficients 2nd ed.,, (2015), 234
|
|
|
Polystyrene Backbone Polymers Consisting of Alkyl-Substituted Triazine Side Groups for Phosphorescent OLEDs. Salert BCD, et al.
Adv. Mater. Sci. Eng. , (2012)
|