Bromocyclohexane
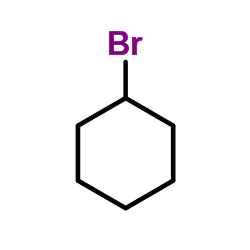
Bromocyclohexane structure
|
Common Name | Bromocyclohexane | ||
|---|---|---|---|---|
| CAS Number | 108-85-0 | Molecular Weight | 163.055 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 161.1±9.0 °C at 760 mmHg | |
| Molecular Formula | C6H11Br | Melting Point | -57°C | |
| MSDS | Chinese USA | Flash Point | 62.8±0.0 °C | |
|
Charging of poly(methyl methacrylate) (PMMA) colloids in cyclohexyl bromide: locking, size dependence, and particle mixtures.
Langmuir 31(1) , 65-75, (2015) We studied suspensions of sterically stabilized poly(methyl methacrylate) (PMMA) particles in the solvent cyclohexyl bromide (CHB; εr = 7.92). We performed microelectrophoresis measurements on suspensions containing a single particle species and on binary mix... |
|
|
Fabrication of polyhedral particles from spherical colloids and their self-assembly into rotator phases.
Angew. Chem. Int. Ed. Engl. 53(50) , 13830-4, (2014) Particle shape is a critical parameter that plays an important role in self-assembly, for example, in designing targeted complex structures with desired properties. Over the last decades, an unprecedented range of monodisperse nanoparticle systems with contro... |
|
|
Isolation of axial conformers of chloro- and bromocyclohexane in a pure state as inclusion complexes with 9,9'-bianthryl, and the discovery of a novel 1,3 diaxial Cl...H weak interaction.
Chem. Commun. (Camb.) (29) , 3646-8, (2005) The axial conformers of chloro- and bromocyclohexane were isolated in a pure state as inclusion complexes with 9,9'-bianthryl, and a 1,3 diaxial Cl...H weak interaction was discovered by X-ray analysis of the axial conformer of chlorocyclohexane. |
|
|
Conformer-specific ionization spectroscopy of bromocyclohexane: equatorial versus axial conformers.
J. Phys. Chem. A 114(37) , 10005-10, (2010) Ionization of equatorial and axial conformational isomers of the chair-bromocyclohexane is investigated with use of the vacuum ultraviolet mass-analyzed threshold ionization (MATI) spectroscopic technique. Two distinct ionization energies of 9.8308 ± 0.0025 a... |
|
|
Alkylation on graphite in the absence of Lewis acids. Sereda GA.
Tetrahedron Lett. 45(39) , 7265-7267, (2004)
|