16-Dehydropregenolone Acetate
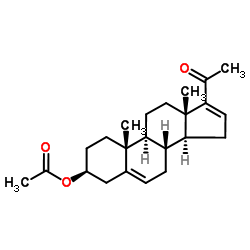
16-Dehydropregenolone Acetate structure
|
Common Name | 16-Dehydropregenolone Acetate | ||
|---|---|---|---|---|
| CAS Number | 979-02-2 | Molecular Weight | 356.498 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 464.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C23H32O3 | Melting Point | 170-178ºC | |
| MSDS | Chinese USA | Flash Point | 200.1±28.8 °C | |
|
[Acylhydrazones of 20-keto steroids and their transformations: I. Synthesis and properties of 1'-acyl-substituted 3'-methylandrosteno[16,17-d]pyrazolines].
Bioorg. Khim. 33(3) , 337-41, (2007) Acetates of 3beta-hydroxy-3'-methyl-1'(N)-acylandrost-5-eno[16,17-d]pyrazolines bearing monothiooxamide acyl groups were synthesized during the study of approaches to the synthesis of 3'-methylandrosteno[16,17-d]azoles, promising biologically active analogues... |
|
|
Pregna-D'-pentaranes - a new class of active gestagenes.
J. Steroid Biochem. 16(1) , 61-7, (1982) A new class of modified progesterones with an additional ring in the 16 alpha , 17 alpha-position (pregna-D'-pentaranes) are described. Compounds containing 4- and 6-membered D'-ring (D'4- and D'6-pentaranes) were synthesized by the cycloaddition of acetylene... |
|
|
Metabolism of steroid acetates by Streptomyces albus.
J. Steroid Biochem. 20(3) , 781-4, (1984) Fermentation of 16-dehydropregnenolone acetate (1a) with Streptomyces albus yielded 16-dehydropregnenolone (1b) and 16-dehydroprogesterone (IIa). Similar incubation of pregnenolone acetate (Ic) with the strain afforded pregnenolone (Id), progesterone (IIb) an... |
|
|
New 5alpha-reductase inhibitors: in vitro and in vivo effects.
Steroids 70(3) , 217-24, (2005) The enzyme 5alpha-reductase is responsible for the conversion of testosterone (T) to its more potent androgen dihydrotestosterone (DHT). This steroid had been implicated in androgen-dependent diseases such as: benign prostatic hyperplasia, prostate cancer, ac... |
|
|
Oxidative side-chain and ring fission of pregnanes by Arthrobacter simplex.
Biochem. J. 255(3) , 769-74, (1988) Metabolic processes involving side-chain and ring cleavage of progesterone, 17-hydroxyprogesterone, 11-deoxycortisol and 16-dehydropregnenolone by Arthrobacter simplex were studied. The formation of the metabolites from progesterone indicates a pathway somewh... |