4-Cyanophenylboronic acid
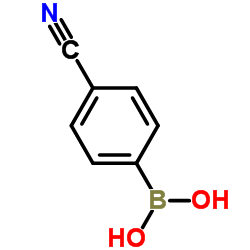
4-Cyanophenylboronic acid structure
|
Common Name | 4-Cyanophenylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 126747-14-6 | Molecular Weight | 146.94 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 355.9±44.0 °C at 760 mmHg | |
| Molecular Formula | C7H6BNO2 | Melting Point | >350 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 169.0±28.4 °C | |
|
Use of dimethyl carbonate as a solvent greatly enhances the biaryl coupling of aryl iodides and organoboron reagents without adding any transition metal catalysts.
Chem. Commun. (Camb.) 23th ed., 48 , 2912-2914, (2012) The coupling reaction of aryl iodides with arylboronic acids to give biaryl compounds can be efficiently performed without adding a transition metal catalyst. The key to success is the use of dimethyl carbonate as a solvent. This finding provides a new strate... |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combinations of N-(2-pyridyl) substituted benzylamines and arylbo... |
|
|
3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential.
J. Med. Chem. 7th ed., 55 , 3479-3487, (2012) A novel class of orally active antimalarial 3,5-diaryl-2-aminopyridines has been identified from phenotypic whole cell high-throughput screening of a commercially available SoftFocus kinase library. The compounds were evaluated in vitro for their antiplasmodi... |
|
|
Fullerenyl boronic esters: ferric perchlorate-mediated synthesis and functionalization.
Org. Lett. 7th ed., 14 , 1800-1803, (2012) Fullerenyl boronic esters have been prepared by a ferric perchlorate-promoted reaction of [60]fullerene with various arylboronic acids. The obtained fullerenyl boronic esters could undergo further functionalization with diols to afford C(60)-fused dioxane/dio... |
|
|
Phosphine-free Suzuki-Miyaura cross-coupling in aqueous media enables access to 2-C-aryl-glycosides.
Org. Lett. 7th ed., 14 , 1728-1731, (2012) A general strategy for the synthesis of 2-aryl-glycals and their elaboration to 2-C-aryl-α-glycosides and 1,5-anhydro-2-C-aryl-2-deoxy alditols are described. The use of reliable, efficient phosphine-free Suzuki-Miyaura cross-coupling of 2-iodoglycals in aque... |
|
|
Synthesis of new trisulfonated calix[4]arenes functionalized at the upper rim, and their complexation with the trimethyllysine epigenetic mark.
Org. Lett. 6th ed., 14 , 1512-1515, (2012) A synthetic route to produce a new family of trisulfonated calix[4]arenes bearing a single group, selectively introduced, that lines the binding pocket is reported. Ten examples, including new sulfonamide and biphenyl-substituted hosts, each with additional b... |
|
|
Ligand-free copper-catalyzed coupling of nitroarenes with arylboronic acids Zhang, J.; et al.
Green Chem. 4th ed., 14 , 912-916, (2012)
|
|
|
Highly effective synthesis of C-5-substituted 2'-deoxyuridine using Suzuki-Miyaura cross-coupling in water Sartori, G.; et al.
Synthesis 5th ed., 44 , 767-772, (2012)
|
|
|
Chan-Lam-type S-arylation of thiols with boronic acids at room temperature.
J. Org. Chem. 6th ed., 77 , 2878-2884, (2012) In this work, an efficient CuSO(4)-catalyzed S-arylation of thiols with aryl and heteroaryl boronic acids at room temperature is established. This catalytic system can tolerate a wide variety of thiols and arylboronic acids in the presence of only 5 mol % of ... |
|
|
Novel dicationic imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines as antiprotozoal agents.
J. Med. Chem. 47 , 3658-3664, (2004) 2-[5-(4-Amidinophenyl)-furan-2-yl]-5,6,7,8-tetrahydro-imidazo[1,2-a]pyridine-6-carboxamidine acetate salt (7) was synthesized from 2-[5-(4-cyanophenyl)-furan-2-yl]-imidazo[1,2-a]pyridine-6-carbonitrile (4a), through the bis-O-acetoxyamidoxime followed by hydr... |