2-Bromothiophene-5-Sulfonamide
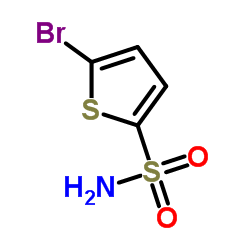
2-Bromothiophene-5-Sulfonamide structure
|
Common Name | 2-Bromothiophene-5-Sulfonamide | ||
|---|---|---|---|---|
| CAS Number | 53595-65-6 | Molecular Weight | 242.114 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 386.4±52.0 °C at 760 mmHg | |
| Molecular Formula | C4H4BrNO2S2 | Melting Point | 138-142 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 187.5±30.7 °C | |
|
Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties.
Molecules 20 , 19914-28, (2015) A variety of novel 5-aryl thiophenes 4a-g containing sulphonylacetamide (sulfacetamide) groups were synthesized in appreciable yields via Pd[0] Suzuki cross coupling reactions. The structures of these newly synthesized compounds were determined using spectral... |
|
|
Cerebrovasodilatation through selective inhibition of the enzyme carbonic anhydrase. 3. 5-(Arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides.
J. Med. Chem. 24(8) , 959-64, (1981) A series of 5-(arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides is described and anticonvulsant activities are listed for the compounds. In most cases, the sulfones had the highest activity and the sulfides the least. Sulfones with ... |
|
|
Acyl sulfonamide anti-proliferatives. Part 2: activity of heterocyclic sulfonamide derivatives.
Bioorg. Med. Chem. Lett. 15(3) , 617-20, (2005) The anti-proliferative activity of acylated heterocyclic sulfonamides is described in Vascular Endothelial Growth Factor-dependent Human Umbilical Vascular Endothelial Cells (VEGF-HUVEC) and in HCT116 tumor cells in a soft agar diffusion assay. |
|
|
Development of an Acyl Sulfonamide Anti-Proliferative Agent, LY573636· Na†. Yates MH, et al.
Org. Process Res. Dev. 13(2) , 255-262, (2009)
|
|
|
A facile synthesis of new 5-aryl-thiophenes bearing sulfonamide moiety via Pd (0)-catalyzed Suzuki-Miyaura cross coupling reactions and 5-bromothiophene-2-acetamide: As potent urease inhibitor, antibacterial agent and hemolytically active compounds. Noreen M, et al.
J. Saudi Chem. Soc. , (2014)
|