1H-Imidazole hydrochloride
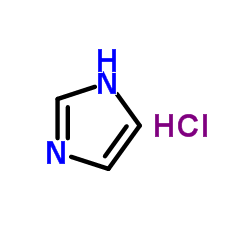
1H-Imidazole hydrochloride structure
|
Common Name | 1H-Imidazole hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1467-16-9 | Molecular Weight | 104.538 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C3H5ClN2 | Melting Point | 158-161 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Covalent bonding of zeolitic imidazolate framework-90 to functionalized silica fibers for solid-phase microextraction.
Chem. Commun. (Camb.) 49(21) , 2142-4, (2013) Here we report a covalent bonding approach to fabricate a robust metal-organic framework ZIF-90 coating for solid-phase microextraction. The ZIF-90 bonded fiber exhibits high enhancement factors, wide linearity, excellent reproducibility, and good lifetime fo... |
|
|
[Reactive oxygen forms and Ca ions as possible intermediaries under the induction of heat resistance of plant cells by jasmonic acid].
Ukr. Biokhim. Zh. 85(3) , 62-8, (2013) The participation of reactive oxygen species (ROS) and calcium ions in realization of influence of exogenous jasmonic acid (JA) on the heat resistance of wheat coleoptiles has been investigated. Influence of 1 microM JA caused the transitional intensifying of... |
|
|
Identification of a pKa-regulating motif stabilizing imidazole-modified double-stranded DNA.
Nucleic Acids Res. 43(1) , 51-62, (2015) The predictable 3D structure of double-stranded DNA renders it ideally suited as a template for the bottom-up design of functionalized nucleic acid-based active sites. We here explore the use of a 14mer DNA duplex as a scaffold for the precise and predictable... |
|
|
Organic-inorganic hybrids of imidazole complexes of zinc (II) for catalysts in the glycerolysis of urea.
J. Nanosci. Nanotechnol. 14(6) , 4632-8, (2014) Bis(alkylimidazole) complexes of zinc, (RIm)2ZnX2, were prepared by a metal insertion reaction. The synthesized (RIm)2ZnX2 exhibited good catalytic performance during synthesis of glycerol carbonate (GC) from glycerol and urea. (HEIm)2ZnCl2 with a hydroxyl gr... |
|
|
Fluorogenic dansyl-ligated gold nanoparticles for the detection of sulfur mustard by displacement assay.
Chem. Commun. (Camb.) 49(23) , 2293-5, (2013) The dansyl fluorophore ligated to gold nanoparticles via imidazole and amine groups affords conjugates capable of detecting micromolar concentrations of the chemical warfare agent sulfur mustard by a fluorescence switching 'ON' displacement assay. |
|
|
Electrocatalytic O2 reduction reaction by synthetic analogues of cytochrome P450 and myoglobin: in-situ resonance Raman and dynamic electrochemistry investigations.
Inorg. Chem. 52(17) , 9897-907, (2013) Bioinspired electrodes have been constructed by physiabsorption of two air stable iron porphyrin complexes, one bearing an imidazole coordination and the other bearing a thiolate coordination. To control the electron transfer (ET) rate to these O2 reducing el... |
|
|
Animal toxicity of hairpin pyrrole-imidazole polyamides varies with the turn unit.
J. Med. Chem. 56(18) , 7449-57, (2013) A hairpin pyrrole-imidazole polyamide (1) targeted to the androgen receptor consensus half-site was found to exert antitumor effects against prostate cancer xenografts. A previous animal study showed that 1, which has a chiral amine at the α-position of the γ... |
|
|
Spectroscopic studies on the interactions between imidazolium chloride ionic liquids and bovine serum albumin.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 104 , 377-82, (2013) The binding of three imidazolium chloride ionic liquids (ILs) with bovine serum albumin (BSA) were investigated by UV absorption spectra, fluorescence spectra and synchronous fluorescence spectra. The results showed that the UV absorption of the BSA was red-s... |
|
|
Noncovalent interactions in halogenated ionic liquids: theoretical study and crystallographic implications.
Phys. Chem. Chem. Phys. 15(12) , 4405-14, (2013) In recent years, several specific imidazolium-based ionic liquids with halogen substituents on the imidazole ring as well as on the alkyl chains have been reported. In this work, noncovalent interactions in four halogenated ionic liquids, i.e. 2-bromo-/iodo- ... |
|
|
Dissolution and depolymerization of barley starch in selected ionic liquids.
Carbohydr. Polym. 93(1) , 89-94, (2013) Polysaccharides like starch are poorly soluble in common solvents. However, certain ionic liquids (ILs) have been found to dissolve them, although some depolymerization happens during the dissolution. Dissolution and depolymerization of barley starch in ten i... |