Arbutin
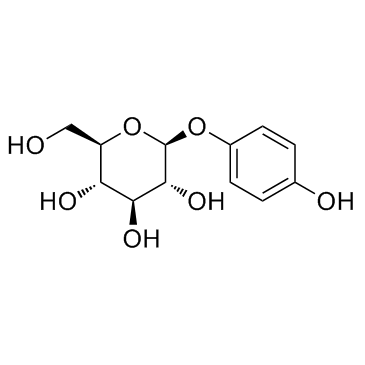
Arbutin structure
|
Common Name | Arbutin | ||
|---|---|---|---|---|
| CAS Number | 497-76-7 | Molecular Weight | 272.251 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 561.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C12H16O7 | Melting Point | 195-198 °C | |
| MSDS | Chinese USA | Flash Point | 293.4±30.1 °C | |
|
Change in chemical constituents and free radical-scavenging activity during Pear (Pyrus pyrifolia) cultivar fruit development.
Biosci. Biotechnol. Biochem. 79(2) , 260-70, (2015) Changes in chemical constituent contents and DPPH radical-scavenging activity in fruits of pear (Pyrus pyrifolia) cultivars during the development were investigated. The fruits of seven cultivars (cv. Niitaka, Chuhwangbae, Wonhwang, Hwangkeumbae, Hwasan, Manp... |
|
|
Cellular Anti-Melanogenic Effects of a Euryale ferox Seed Extract Ethyl Acetate Fraction via the Lysosomal Degradation Machinery.
Int. J. Mol. Sci. 16 , 9217-35, (2015) The aim of this study was to investigate the effect of ethyl acetate fraction of Euryale ferox seed extracts (Efse-EA) on melanogenesis in immortalized mouse melanocyte cell line, melan-a. Efse-EA showed strong dose-dependent mushroom tyrosinase inhibitory ac... |
|
|
Avocado Proanthocyanidins as a Source of Tyrosinase Inhibitors: Structure Characterization, Inhibitory Activity, and Mechanism.
J. Agric. Food Chem. 63 , 7381-7, (2015) Proanthocyanidins were purified from avocado (Persea americana) fruit, and their structures were analyzed by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) and high-performance liquid chromatography-electrospray io... |
|
|
Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: Effect of temperature and pH on decomposition.
Int. J. Cosmet. Sci. 37 , 567-73, (2015) Arbutin is an effective agent for the treatment of melanin disorders. Arbutin may be converted to hydroquinone under conditions of high temperature, ultraviolet (UV) radiation and dilute acid. The aim of the current study was to develop an analytical method t... |
|
|
Anti-melanogenic effect of ginsenoside Rg3 through ERK-mediated inhibition of microphthalmia-associated transcription factor
J. Ginseng. Res. 39 , 238-42, (2015) Background Panax ginseng has been used to prolong longevity and is believed to be useful for skin beauty. Ginsenosides are the most active components isolated from ginseng, and ginsenoside Rg3 (G-Rg3) in particular has been demonstrated to possess antioxidati... |
|
|
Phytochemical composition of polar fraction of Stachys germanica L. subsp. salviifolia (Ten.) Gams, a typical plant of Majella National Park.
Nat. Prod. Res. 27(2) , 190-3, (2013) In this study, we report the isolation and identification of several compounds present in the polar fraction of Stachys germanica L. subsp. salviifolia (Ten.) Gams, collected in the protected area of Majella National Park. In particular, we have isolated and ... |
|
|
In vitro melanogenesis inhibitory effects of N-feruloyldopamine.
J. Cosmet. Sci. 64(2) , 133-44, (2013) Tyrosinase is the rate-limiting enzyme in the melanogenesis process. It remains the most efficient way to downregulate melanin production and improve unsightly pigmentary disorders. The aim of our investigations was to find a structurally characterized molecu... |
|
|
4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation.
J. Eur. Acad. Dermatol. Venereol. 27 Suppl 1 , 19-23, (2013) Hyperpigmentary disorders like melasma, actinic and senile lentigines are a major cosmetic concern. Therefore, many topical products are available, containing various active ingredients aiming to reduce melanin production and distribution. The most prominent ... |
|
|
Topical treatment of hyperpigmentation disorders.
Ann. Dermatol. Venereol. 139 Suppl 4 , S153-8, (2012) Hyperpigmentation has traditionally been a relatively difficult condition to treat, especially in darker racial ethnic groups. Multiple topical agents available act upon different steps of the pigmentation pathway. We review these topical agents, their mechan... |
|
|
DeoxyArbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis.
Journal. of. Drugs in. Dermatology. 11(10) , e28-34, (2012) Safety is a major concern in developing commercial skin-lightening agents. Here, we report the modulating effects of deoxyArbutin (dA) and its second-generation derivatives - deoxyFuran (dF), 2-fluorodeoxyArbutin (fdA), and thiodeoxyArbutin (tdA) - on tyrosin... |