Germanium(II) sulfide
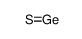
Germanium(II) sulfide structure
|
Common Name | Germanium(II) sulfide | ||
|---|---|---|---|---|
| CAS Number | 12025-32-0 | Molecular Weight | 107.72900 | |
| Density | 4.100 | Boiling Point | 1503.99°C (estimate) | |
| Molecular Formula | GeH3S | Melting Point | 615ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Germanium sulfide(II and IV) nanoparticles for enhanced performance of lithium ion batteries.
Chem. Commun. (Camb.) 49(41) , 4661-3, (2013) Germanium sulfide (GeS and GeS2) nanoparticles were synthesized by novel gas-phase laser photolysis and subsequent thermal annealing. They showed excellent cycling performance for lithium ion batteries, with a maximum capacity of 1010 mA h g(-1) after 100 cyc... |
|
|
Electrochemical deposition of germanium sulfide from room-temperature ionic liquids and subsequent Ag doping in an aqueous solution.
Langmuir 28(13) , 5513-7, (2012) A facile room-temperature electrochemical deposition process for germanium sulfide (GeS(x)) has been developed with the use of an ionic liquid as an electrolyte. The electrodeposition mechanism follows the induced codeposition of Ge and S precursors in ionic ... |
|
|
Small germanium sulfide clusters: mass spectrometry and density functional calculations.
Phys. Chem. Chem. Phys. 12(30) , 8557-63, (2010) Germanium sulfide clusters were generated by laser ablation of a solid sample. The resulting molecules were analyzed in a time-of-flight mass spectrometer. In addition to atomic germanium and diatomic sulfur, the spectra exhibited evidence for the existence o... |
|
|
Thermal analysis of germanium (II) sulfide." Ross L and Bourgon M
Can. J. Chem. 46(14) , 2464-2468, (1968)
|