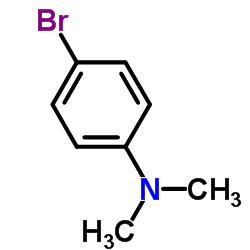
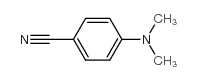
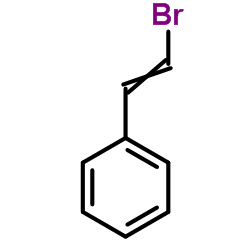
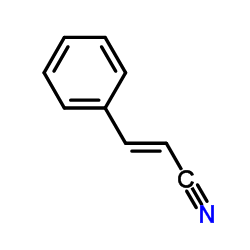
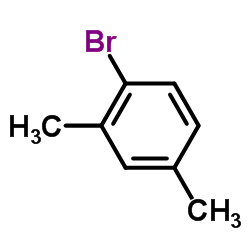
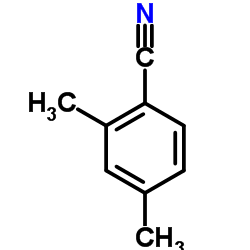
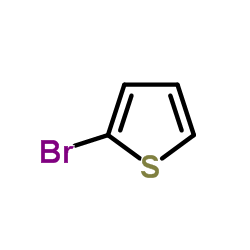
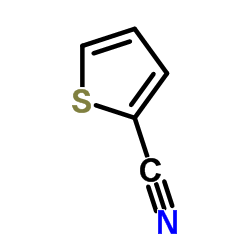
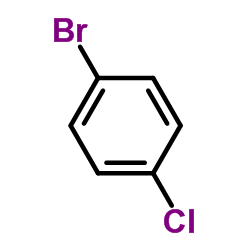
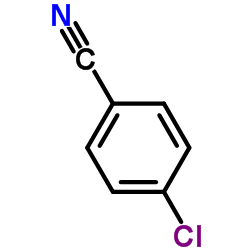
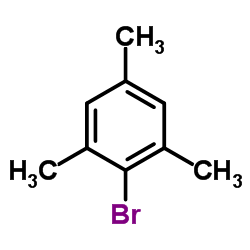
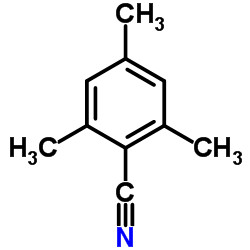
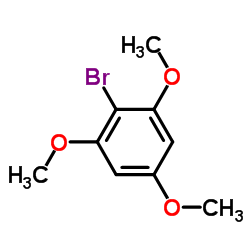
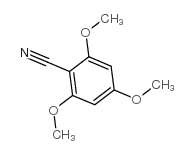
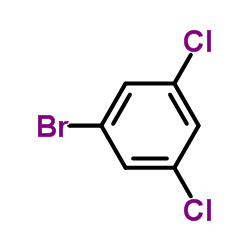
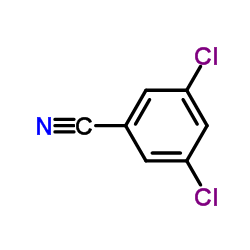
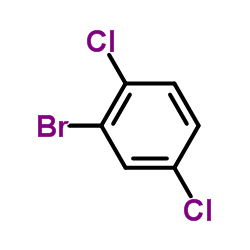
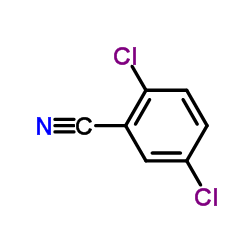
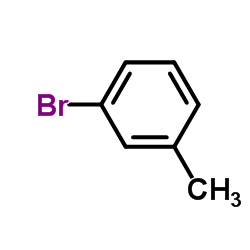
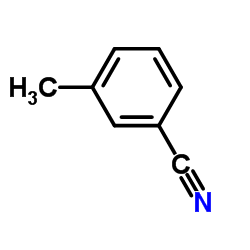
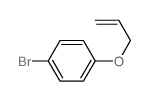
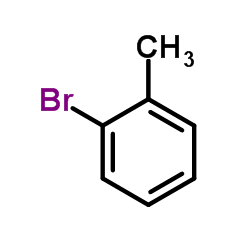
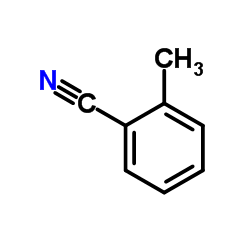
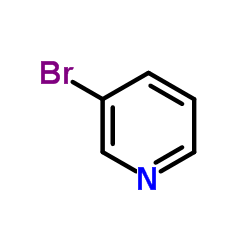
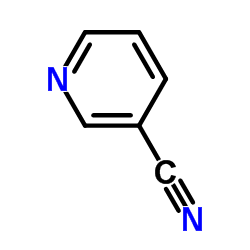
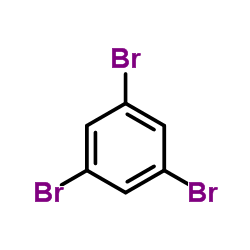
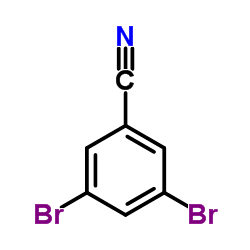
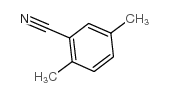
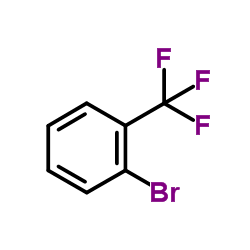
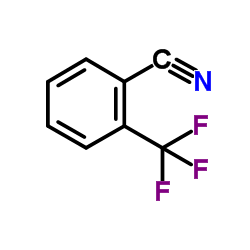
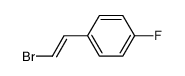
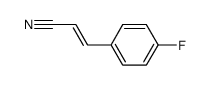
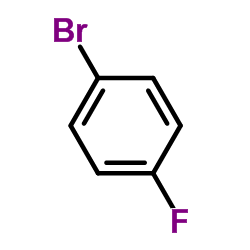
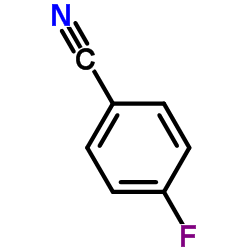
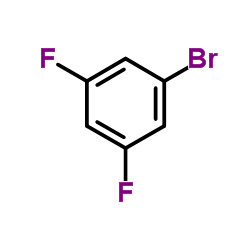
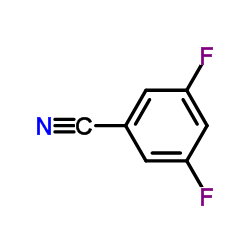
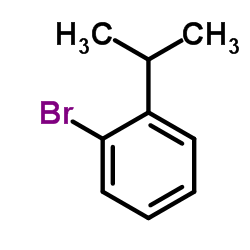
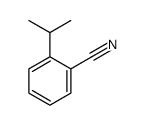
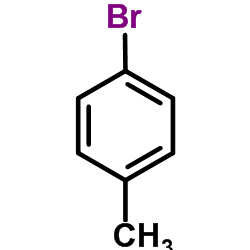
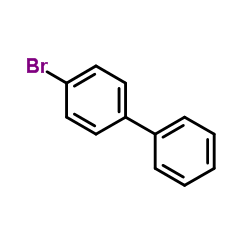
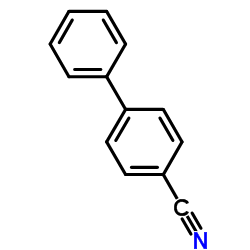
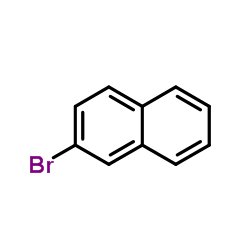
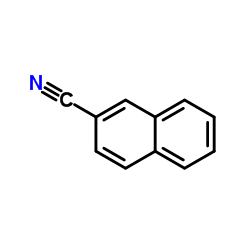
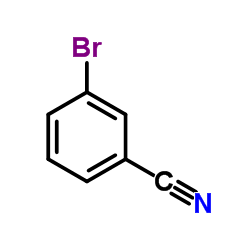
|
~62% |
|
~85% |
|
~60% |
|
~69% |
|
~59% |
|
~78% |
|
~62% |
|
~72% |
|
~74% |
|
~73% |
|
~69% |
|
~65% |
|
~50% |
|
~76% |
|
~81% |
|
~62% |
|
~52% |
|
~62% |
|
~68% |
|
~82% |
|
~65% |
|
~83% |
|
~63% |
|
~63% |
|
~70% |