| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
sucrose
CAS:57-50-1 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
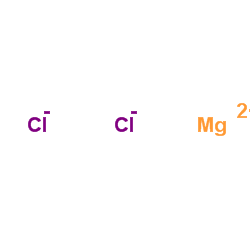 |
Magnesium choride
CAS:7786-30-3 |
|
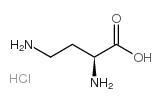 |
H-Dab.HCl
CAS:1482-98-0 |
|
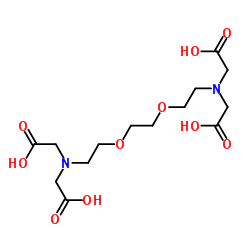 |
EGTA
CAS:67-42-5 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
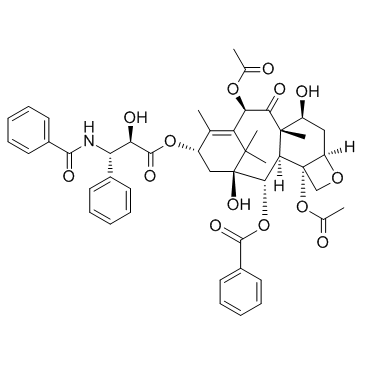 |
Paclitaxel
CAS:33069-62-4 |
|
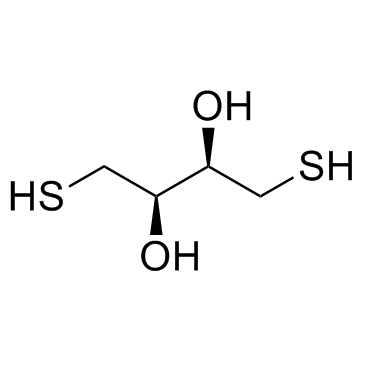 |
DL-Dithiothreitol
CAS:3483-12-3 |