Paclitaxel
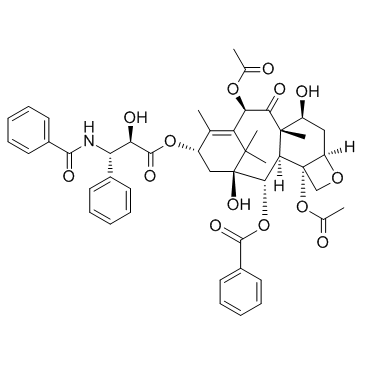
Paclitaxel structure
|
Common Name | Paclitaxel | ||
|---|---|---|---|---|
| CAS Number | 33069-62-4 | Molecular Weight | 853.906 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 957.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C47H51NO14 | Melting Point | 213 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 532.6±34.3 °C | |
| Symbol |



GHS05, GHS07, GHS08 |
Signal Word | Danger | |
Use of PaclitaxelPaclitaxel is a potent anticancer medication which can promote microtubule (MT) assembly, inhibit MT depolymerization, and change MT dynamics required for mitosis and cell proliferation. |
| Name | paclitaxel |
|---|---|
| Synonym | More Synonyms |
| Description | Paclitaxel is a potent anticancer medication which can promote microtubule (MT) assembly, inhibit MT depolymerization, and change MT dynamics required for mitosis and cell proliferation. |
|---|---|
| Related Catalog | |
| Target |
IC50: 4 nM (MT) |
| In Vitro | Paclitaxel at 0.1, 0.5, and 1 μM reduces the proliferation and survival of CCRF-HSB-2 cells in a dose-dependent fashion and that the IC50 value of taxol is about 0.25 μM[1]. Paclitaxel directly associates with the endoplasmic reticulum to stimulate the release of calcium into the cytosol, contributing to the induction of apoptosis[2]. |
| In Vivo | In a SCID mouse xenograft model, low dose metronomic Paclitaxel treatment decreases lung dissemination of EGI-1 cells without significantly affecting their local tumor growth[3]. Low doses of paclitaxel promot liver metastasis in mouse xenografts, which correlats with changes in estrogen metabolism in the host liver[4]. Paclitaxel (2 mg/kg per treatment, black circles) induces mechanical hypersensitivity in the glabrous skin of the hindpaw[5]. |
| Kinase Assay | To determine which caspases are involved in apoptosis induced by taxol, caspase-3 inhibitor (DEVD-CHO), caspase-6 inhibitor (Z-VEID-FMK), caspase-8 inhibitor (Z-IETD-FMK or IETD-CHO), caspase-9 inhibitors (Z-LEHD-FMK or LEHD-CHO), and caspase-10 inhibitor (Z-AEVD-FMK) are used. These caspase inhibitors are dissolved in dimethyl sulfoxide (Me2SO); the final concentration of Me2SO is 0.1%. Cells (5×105) are preincubated in the presence or absence of 100 μM each of these inhibitors for 3 h at 37°C then treated with or without 0.1, 0.5, and 1 μM Paclitaxel for 48 h and processed for annexin V binding assay. |
| Cell Assay | 1×104 cells are plated in 100 μL of the growth medium in the presence or absence of increasing concentrations (0.1-1 μM) of taxol in 96-well plates and cultured at 37°C in 5% CO2 for 12-48 h. The cells are then incubated with 25 μL of MTT (5 mg/mL) at 37°C for 4 h. After dissolving the crystals with 0.04 N HCl in isopropanol, the plates are read in a microplate reader at 570 nm. The concentration of drug that inhibits cell survival by 50% (IC50) is determined from cell survival plots. |
| Animal Admin | Adult (250-320 g) male Sprague-Dawley rats are used for all experiments. Rats are housed two per cage in a temperature and humidity controlled, on a 12 h:12 h light:dark schedule with food and water available ad libitum. One week following the DiI injection, rats are anesthetized with isofluorane and injected into the tail vein with 2 mg/kg paclitaxel or its vehicle (1:1:23, cremophor EL:ethanol:0.9% saline). The tail vein injection is repeated three more times every other day for a total of four injections. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 957.1±65.0 °C at 760 mmHg |
| Melting Point | 213 °C (dec.)(lit.) |
| Molecular Formula | C47H51NO14 |
| Molecular Weight | 853.906 |
| Flash Point | 532.6±34.3 °C |
| Exact Mass | 853.330933 |
| PSA | 221.29000 |
| LogP | 7.38 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.637 |
| InChIKey | RCINICONZNJXQF-MZXODVADSA-N |
| SMILES | CC(=O)OC1C(=O)C2(C)C(O)CC3OCC3(OC(C)=O)C2C(OC(=O)c2ccccc2)C2(O)CC(OC(=O)C(O)C(NC(=O)c3ccccc3)c3ccccc3)C(C)=C1C2(C)C |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. Combustible. |
| Water Solubility | methanol: 50 mg/mL, clear, colorless |
| Symbol |



GHS05, GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H317-H318-H334-H335-H341-H361 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P305 + P351 + P338 + P310-P342 + P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R40;R41 |
| Safety Phrases | 22-26-36/37/39-45 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | DA8340700 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2932999021 |
| Precursor 7 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999021 |
|---|
|
Deacetyl-mycoepoxydiene, isolated from plant endophytic fungi Phomosis sp. demonstrates anti-microtubule activity in MCF-7 cells.
Biomed. Pharmacother. 69 , 82-9, (2015) Deacetyl-mycoepoxydiene (DM), a novel secondary metabolite produced by the plant endophytic fungi Phomosis sp., induced the reorganization of cytoskeleton in actively growing MCF-7 cells by promoting ... |
|
|
Cell-cell adhesions and cell contractility are upregulated upon desmosome disruption.
PLoS ONE 9(7) , e101824, (2014) Desmosomes are perturbed in a number of disease states - including genetic disorders, autoimmune and bacterial diseases. Here, we report unexpected changes in other cell-cell adhesion structures upon ... |
|
|
Control of cytoplasmic dynein force production and processivity by its C-terminal domain.
Nat. Commun. 6 , 6206, (2015) Cytoplasmic dynein is a microtubule motor involved in cargo transport, nuclear migration and cell division. Despite structural conservation of the dynein motor domain from yeast to higher eukaryotes, ... |
| (2α,3ξ,5β,7β,10β,13α)-4,10-Diacetoxy-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| BMS 181339-01 |
| Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13 ,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| Capxol |
| Plaxicel |
| Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-t etramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| taxal |
| benzenepropanoic acid, b-(benzoylamino)-a-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (aR,bS)- |
| benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| Onxal |
| Paxceed |
| (2a,5b,7b,10b,13a)-4,10-bis(acetyloxy)-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| (2α,5β,7β,10β,13α)-4,10-Diacetoxy-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| 5b,20-Epoxy-1,2a,4,7b,10b,13a-hexahydroxytax-11-en-9-one 4,10-Diacetate 2-Benzoate 13-Ester with (2R,3S)-N-Benzoyl-3-phenylisoserine |
| Yewtaxan |
| MFCD00869953 |
| EINECS 205-285-7 |
| Paclitaxel |
| TAXUS |
| NK 105 |
| TaxAlbin |
| DHP 107 |
| Peclitaxel |
| Taxol |
| (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-Bis(acetyloxy)-1,9-dihydroxy-15-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0.0]heptadec-13-en-2-ylbenzolcarboxylat |
| Paxene |
| Taxol A |
| LipoPac |
| Ebetaxel |
| ABI 007 |
| (2α,5β,7β,10β,13α)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| Anzatax |
| UNII-P88XT4IS4D |
| (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-bis(acetyloxy)-1,9-dihydroxy-15-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0.0]heptadec-13-en-2-yl benzoate |
| EMPAC |
| Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| Onxol |
![1-hydroxy-7β-triethylsilyloxy-9-oxo-10β-acetyloxy-5β,20-epoxytax-11-ene-2α,4,13α-triyl 4-acetate 2-benzoate 13-[(2R,3S)-3-benzoylamino-2-triethylsilyloxy-3-phenylpropanoate] Structure](https://image.chemsrc.com/caspic/484/135365-62-7.png) CAS#:135365-62-7
CAS#:135365-62-7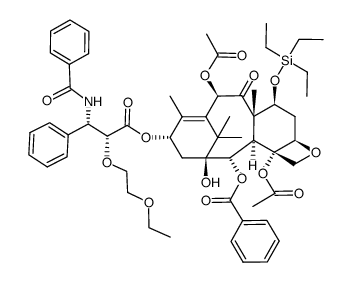 CAS#:439813-57-7
CAS#:439813-57-7![7-(triethylsilyl)-13-O-[((4S,5R)-2,4-diphenyl-4,5-dihydrooxazol-5-yl)carbonyl]baccatin Structure](https://image.chemsrc.com/caspic/045/158722-23-7.png) CAS#:158722-23-7
CAS#:158722-23-7 CAS#:1122-58-3
CAS#:1122-58-3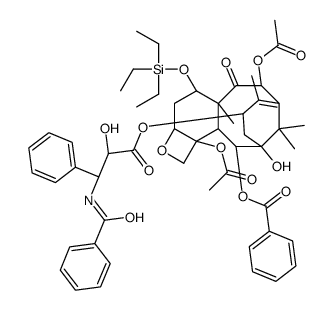 CAS#:148930-55-6
CAS#:148930-55-6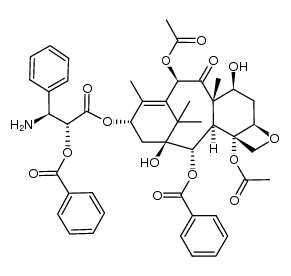 CAS#:307923-51-9
CAS#:307923-51-9 CAS#:718627-80-6
CAS#:718627-80-6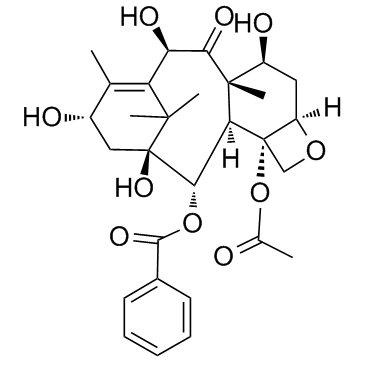 CAS#:32981-86-5
CAS#:32981-86-5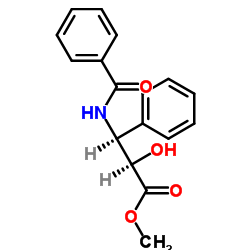 CAS#:32981-85-4
CAS#:32981-85-4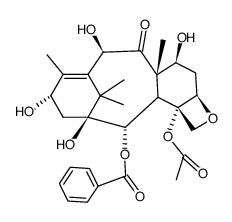 CAS#:71629-92-0
CAS#:71629-92-0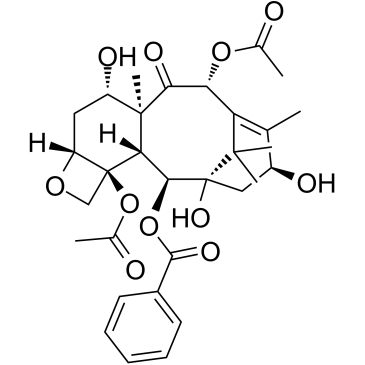 CAS#:27548-93-2
CAS#:27548-93-2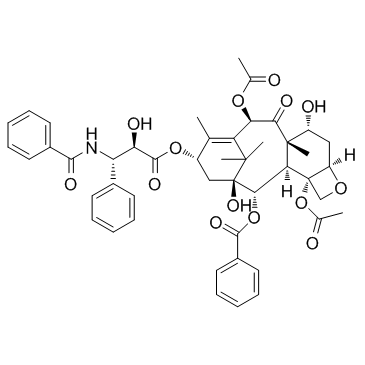 CAS#:105454-04-4
CAS#:105454-04-4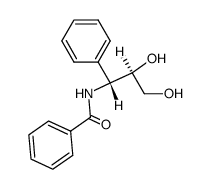 CAS#:103150-32-9
CAS#:103150-32-9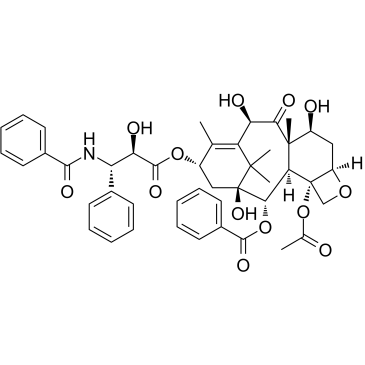 CAS#:78432-77-6
CAS#:78432-77-6![2'-O-{[(2,2,2,-Trichloroethyl)oxy]carbonyl Paclitaxel structure](https://image.chemsrc.com/caspic/003/100431-55-8.png) CAS#:100431-55-8
CAS#:100431-55-8![[2aR-[2aα,4β,4aβ,6β,9α(αR*,βS*),11α,12α,12aα,12bα]]-β-(Benzoylamino)-α-[[(2,2,2-trichloroethoxy)carbonyl]oxy]-benzenepropanoic Acid 6,12b-Bis(acety structure](https://image.chemsrc.com/caspic/221/100449-86-3.png) CAS#:100449-86-3
CAS#:100449-86-3
