| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
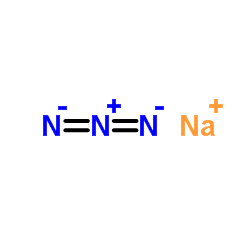 |
Sodium azide
CAS:26628-22-8 |
|
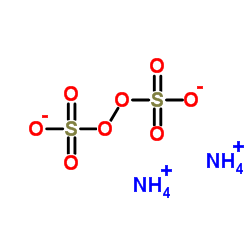 |
ammonium persulfate
CAS:7727-54-0 |
|
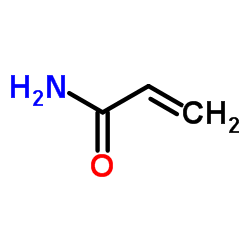 |
Acrylamide Crystals
CAS:79-06-1 |
|
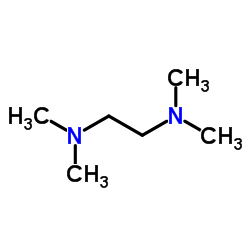 |
TMEDA
CAS:110-18-9 |
|
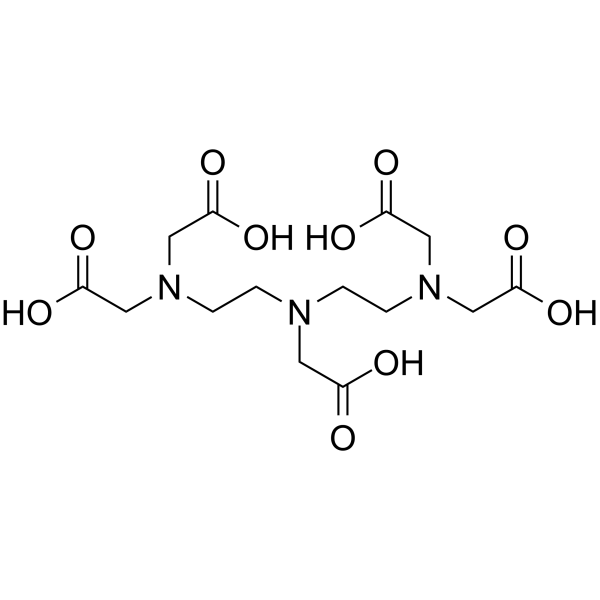 |
Diethylenetriaminepentaacetic acid
CAS:67-43-6 |
|
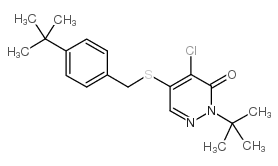 |
Pyridaben
CAS:96489-71-3 |
|
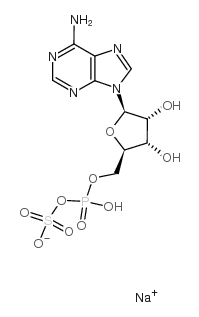 |
APS SODIUM SALT
CAS:102029-95-8 |