| Structure | Name/CAS No. | Articles |
|---|---|---|
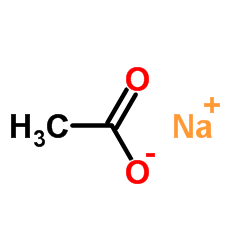 |
Sodium acetate
CAS:127-09-3 |
|
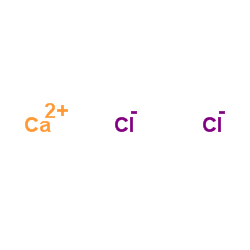 |
Calcium chloride
CAS:10043-52-4 |
|
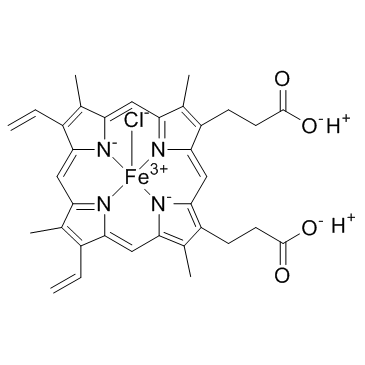 |
Hemin
CAS:16009-13-5 |
|
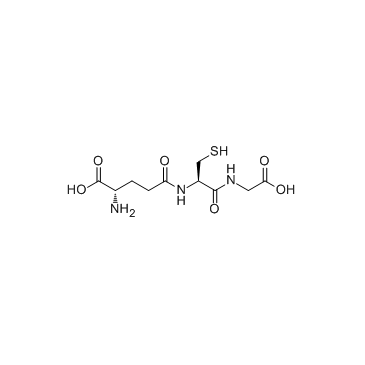 |
Glutathione
CAS:70-18-8 |
|
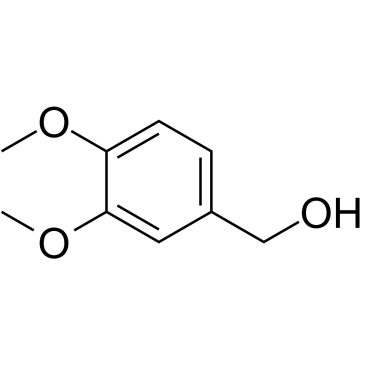 |
3,4-Dimethoxybenzyl Alcohol
CAS:93-03-8 |
|
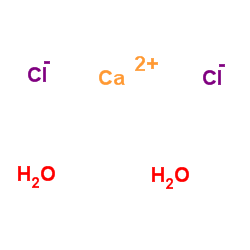 |
calcium chloride dihydrate
CAS:10035-04-8 |