| Structure | Name/CAS No. | Articles |
|---|---|---|
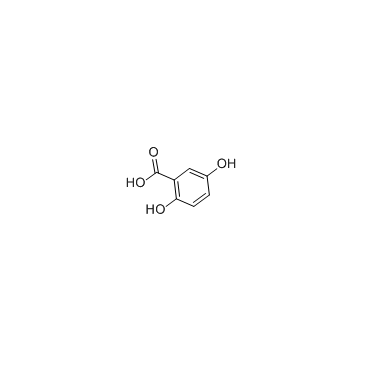 |
Gentisic acid
CAS:490-79-9 |
|
 |
2-Chlorotrityl Chloride
CAS:42074-68-0 |
|
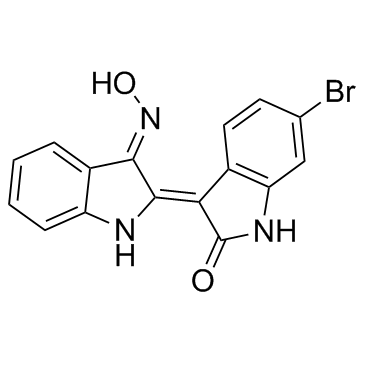 |
BIO
CAS:667463-62-9 |