| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetone
CAS:67-64-1 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
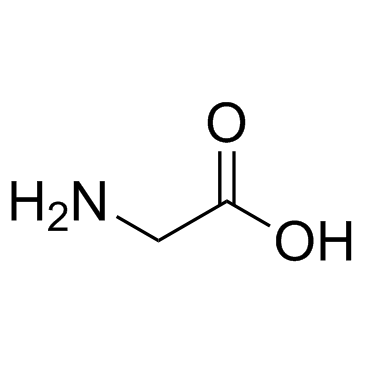 |
Glycine
CAS:56-40-6 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
Sodium deoxycholate
CAS:302-95-4 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
 |
Sodium tetraborate
CAS:1330-43-4 |