| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
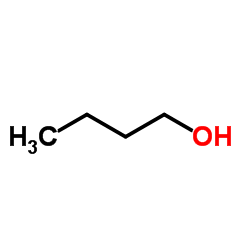 |
Butanol
CAS:71-36-3 |
|
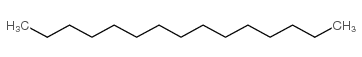 |
pentadecane
CAS:629-62-9 |
|
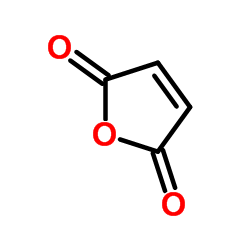 |
Maleic anhydride
CAS:108-31-6 |
|
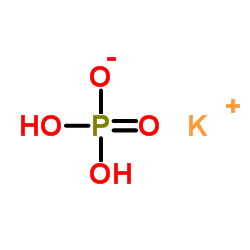 |
Monopotassium phosphate
CAS:7778-77-0 |
|
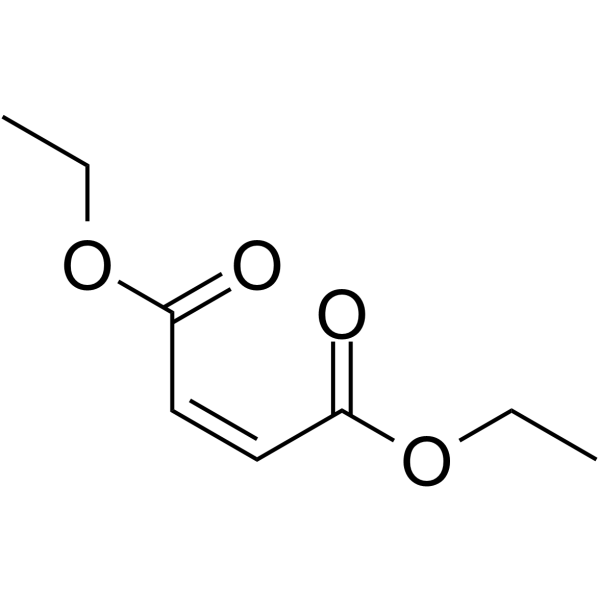 |
diethylmaleate
CAS:141-05-9 |
|
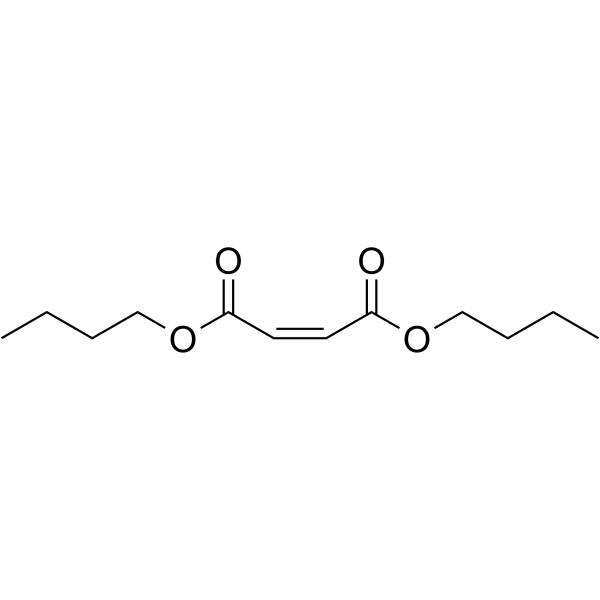 |
n-Butyl fumarate
CAS:105-76-0 |
|
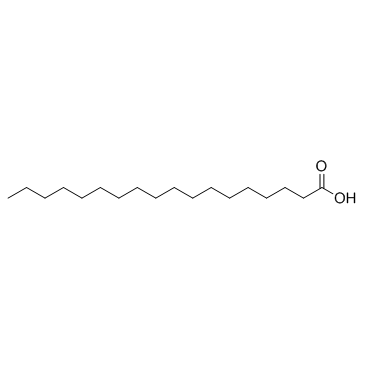 |
stearic acid
CAS:57-11-4 |
|
 |
diethoxymethane
CAS:462-95-3 |