| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L(-)-Tryptophan
CAS:73-22-3 |
|
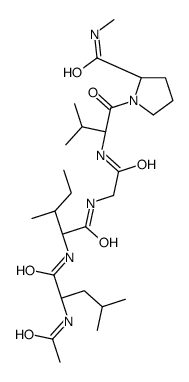 |
Elastin
CAS:9007-58-3 |
|
 |
L-Phenylalanine
CAS:63-91-2 |
|
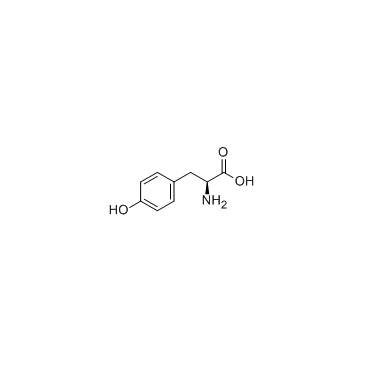 |
L-Tyrosine
CAS:60-18-4 |