| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
2-Amino-2-methyl-1-propanol
CAS:124-68-5 |
|
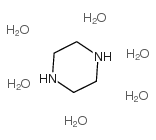 |
Piperazine Hexahydrate
CAS:142-63-2 |
|
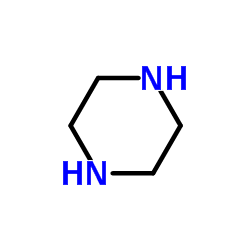 |
Piperazine
CAS:110-85-0 |
|
 |
1-hydroxy-2-methylpropan-2-aminium chloride
CAS:3207-12-3 |