| Structure | Name/CAS No. | Articles |
|---|---|---|
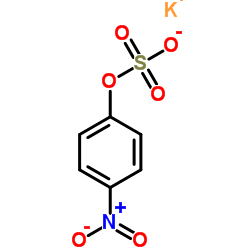 |
Potassium 4-nitrophenyl sulfate
CAS:6217-68-1 |
|
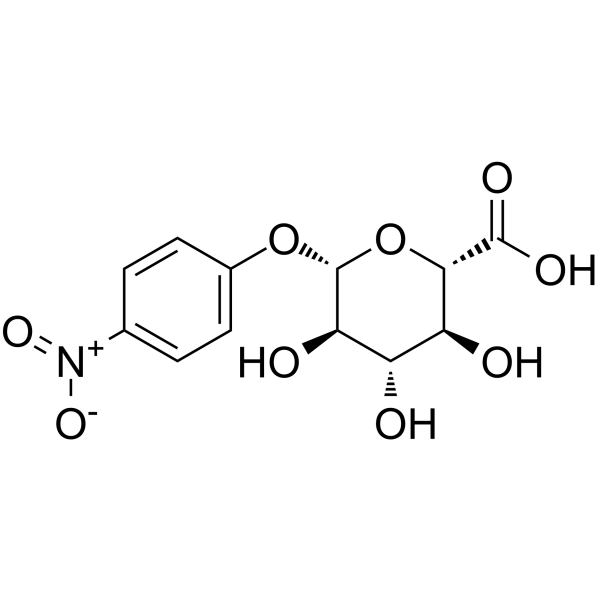 |
4-Nitrophenyl β-D-glucopyranosiduronic acid
CAS:10344-94-2 |