| Structure | Name/CAS No. | Articles |
|---|---|---|
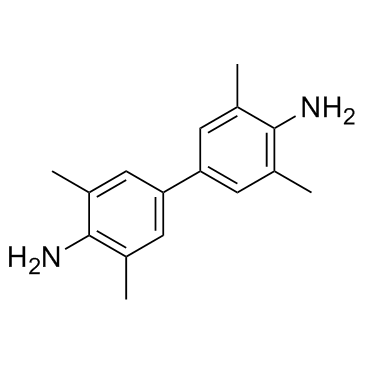 |
Tetramethylbenzidine
CAS:54827-17-7 |
|
 |
Phenol
CAS:108-95-2 |
|
 |
putrescine
CAS:110-60-1 |
|
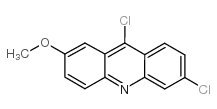 |
6,9-Dichloro-2-methoxyacridine
CAS:86-38-4 |