| Structure | Name/CAS No. | Articles |
|---|---|---|
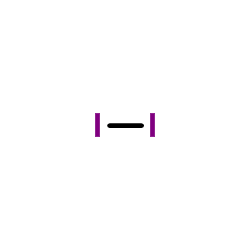 |
molecular iodine
CAS:7553-56-2 |
|
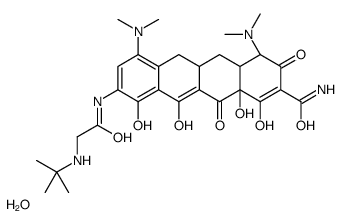 |
Tigecycline hydrate
CAS:1229002-07-6 |
|
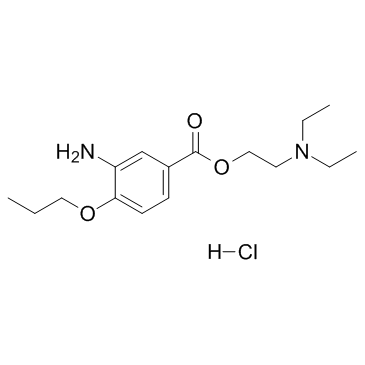 |
Proparacaine Hydrochloride
CAS:5875-06-9 |