| Structure | Name/CAS No. | Articles |
|---|---|---|
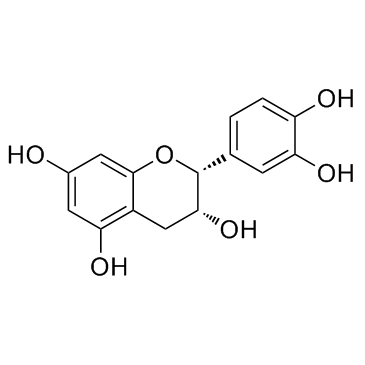 |
Epicatechin
CAS:490-46-0 |
|
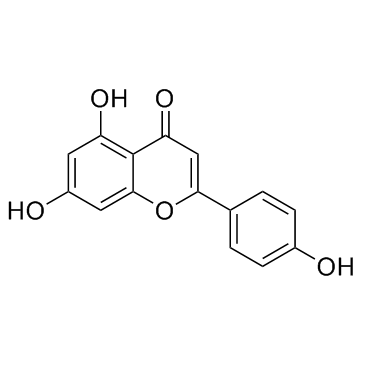 |
Apigenin
CAS:520-36-5 |
|
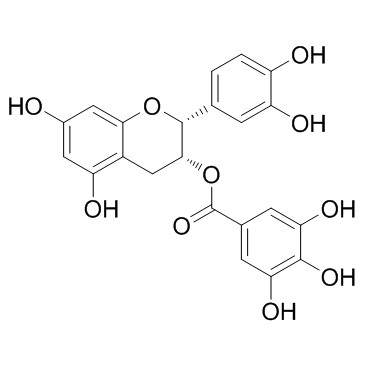 |
(-)-Epicatechin gallate
CAS:1257-08-5 |
|
 |
(-)-Epigallocatechin(EGC)
CAS:970-74-1 |
|
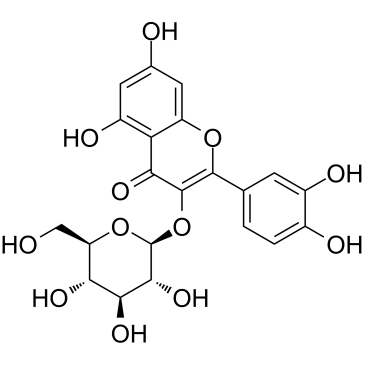 |
Isoquercitrin
CAS:482-35-9 |
|
 |
amentoflavone
CAS:1617-53-4 |
|
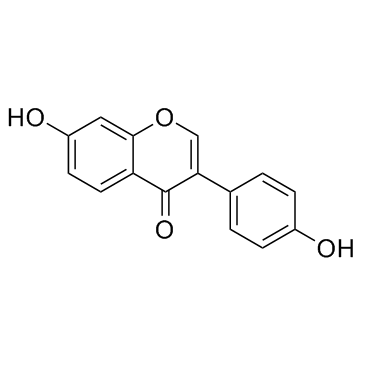 |
Daidzein
CAS:486-66-8 |
|
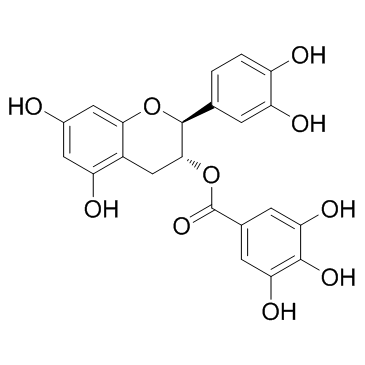 |
(-)-Catechin gallate(CG)
CAS:130405-40-2 |
|
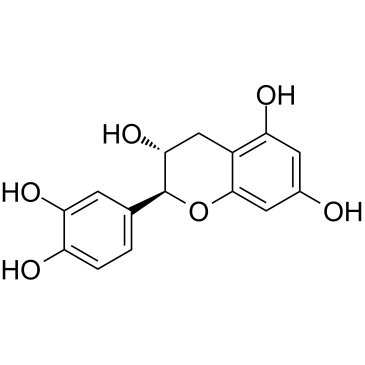 |
(-)-catechin
CAS:18829-70-4 |