Organic Letters
2007-05-10
The Kulinkovich reaction in the synthesis of constrained n,n-dialkyl neurotransmitter analogues.
Catherine A Faler, Madeleine M Joullié
Index: Org. Lett. 9(10) , 1987-90, (2007)
Full Text: HTML
Abstract
An intermolecular Ti(IV)-mediated cyclopropanation reaction has been used to synthesize substituted 2-phenylcyclopropylamines and constrained analogues of the neurotransmitters histamine and tryptamine. Many hydroxy- and methoxy-substituted phenylcyclopropylamines are known to inhibit monoamine oxidase and have been shown to mimic hallucinogens. These compounds were made in 1 to 5 steps from readily available starting materials.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
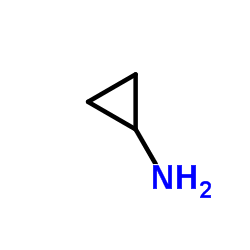 |
Cyclopropanamine
CAS:765-30-0 |
C3H7N |
Related Articles:
More...
|
Synthesis and antibacterial activity of some novel 4-oxopyri...
2014-11-01 [Arch. Pharm. (Weinheim) 347(11) , 861-72, (2014)] |
|
Cyclopropylamines from N,N-dialkylcarboxamides and Grignard ...
2010-12-10 [Chemistry 16 , 13862-13875, (2010)] |
|
Cyclopropylamine inactivation of cytochromes P450: role of m...
2005-04-15 [Arch. Biochem. Biophys. 436(2) , 265-75, (2005)] |
|
Mutation of surface cysteine 374 to alanine in monoamine oxi...
2005-05-16 [Bioorg. Med. Chem. 13(10) , 3487-95, (2005)] |
|
Scalable synthesis of a prostaglandin EP4 receptor antagonis...
2010-06-18 [J. Org. Chem. 75(12) , 4078-85, (2010)] |