Mutation of surface cysteine 374 to alanine in monoamine oxidase A alters substrate turnover and inactivation by cyclopropylamines.
Ana Paula B Vintém, Nigel T Price, Richard B Silverman, Rona R Ramsay
Index: Bioorg. Med. Chem. 13(10) , 3487-95, (2005)
Full Text: HTML
Abstract
Modification of cysteine (Cys) residues inactivates monoamine oxidases (MAO) yet the crystal structure shows no conserved cysteines in the active site of MAO A (Ma, J. et al. J. Mol. Biol.2004, 338, 103-114). MAO A cysteine 374 was mutated to alanine and the purified enzyme characterized kinetically. The mutant was active but had decreased k(cat)/K(m) values compared to the wild-type enzyme. Cyclopropylamine-containing mechanism-based inactivators similarly showed lower turnover rates. Spectral studies and measurement of free thiols established that 1-phenylcyclopropylamine (1-PCPA) formed an irreversible flavin adduct whereas 2-phenylcyclopropylamine (2-PCPA) and N-cyclo-alpha-methylbenzylamine (N-CalphaMBA) formed adducts that allowed reoxidation of the flavin on denaturation and decreased cysteine in both wild-type and mutant MAO A. In the 1-PCPA and N-CalphaMBA inactivations, the partition ratio was decreased by more than 50% in the mutant. The data suggest that mutation of Cys374 influences MAO A catalysis, which has implications for MAO susceptibility to redox damage. These results are compared with previous work on the equivalent residue in MAO B, namely, cysteine 365.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
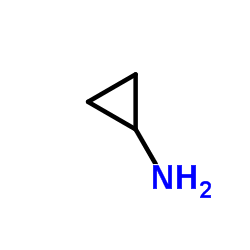 |
Cyclopropanamine
CAS:765-30-0 |
C3H7N |
|
Synthesis and antibacterial activity of some novel 4-oxopyri...
2014-11-01 [Arch. Pharm. (Weinheim) 347(11) , 861-72, (2014)] |
|
Cyclopropylamines from N,N-dialkylcarboxamides and Grignard ...
2010-12-10 [Chemistry 16 , 13862-13875, (2010)] |
|
Cyclopropylamine inactivation of cytochromes P450: role of m...
2005-04-15 [Arch. Biochem. Biophys. 436(2) , 265-75, (2005)] |
|
The Kulinkovich reaction in the synthesis of constrained n,n...
2007-05-10 [Org. Lett. 9(10) , 1987-90, (2007)] |
|
Scalable synthesis of a prostaglandin EP4 receptor antagonis...
2010-06-18 [J. Org. Chem. 75(12) , 4078-85, (2010)] |