Doing the methylene shuffle--further insights into the inhibition of mitotic kinesin Eg5 with S-trityl L-cysteine.
Murad N Abualhasan, James A D Good, Kitiyaporn Wittayanarakul, Nahoum G Anthony, Giacomo Berretta, Oliver Rath, Frank Kozielski, Oliver B Sutcliffe, Simon P Mackay
Index: Eur. J. Med. Chem. 54 , 483-98, (2012)
Full Text: HTML
Abstract
S-Trityl L-cysteine (STLC) is an inhibitor of the mitotic kinesin Eg5 with potential as an antimitotic chemotherapeutic agent. We previously reported the crystal structure of the ligand-protein complex, and now for the first time, have quantified the interactions using a molecular dynamics based approach. Based on these data, we have explored the SAR of the trityl head group using the methylene shuffle strategy to expand the occupation of one of the hydrophobic pockets. The most potent compounds exhibit strong (<100 nM) inhibition of Eg5 in the basal ATPase assay and inhibit growth in a variety of tumour-derived cell lines.Copyright © 2012 Elsevier Masson SAS. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
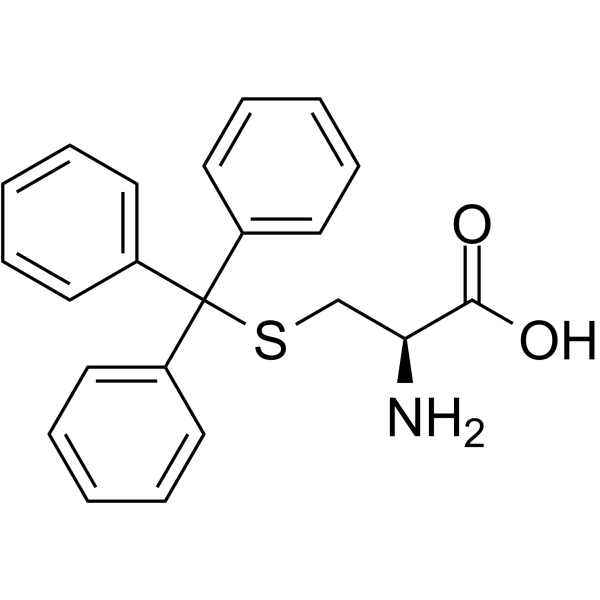 |
S-Tritylcysteine
CAS:2799-07-7 |
C22H21NO2S |
|
Cdk1 phosphorylates the Rac activator Tiam1 to activate cent...
2015-01-01 [Nat. Commun. 6 , 7437, (2015)] |
|
Structure-activity relationship of S-trityl-L-cysteine analo...
2008-03-13 [J. Med. Chem. 51 , 1115-25, (2008)] |
|
Dynamics of Human Telomerase Holoenzyme Assembly and Subunit...
2015-08-28 [J. Biol. Chem. 290 , 21320-35, (2015)] |
|
Nuclear translocation of Cyclin B1 marks the restriction poi...
2014-01-01 [Cell Cycle 13(17) , 2733-43, (2014)] |
|
EGF-induced centrosome separation promotes mitotic progressi...
2013-05-13 [Dev. Cell 25(3) , 229-40, (2013)] |