S-Tritylcysteine
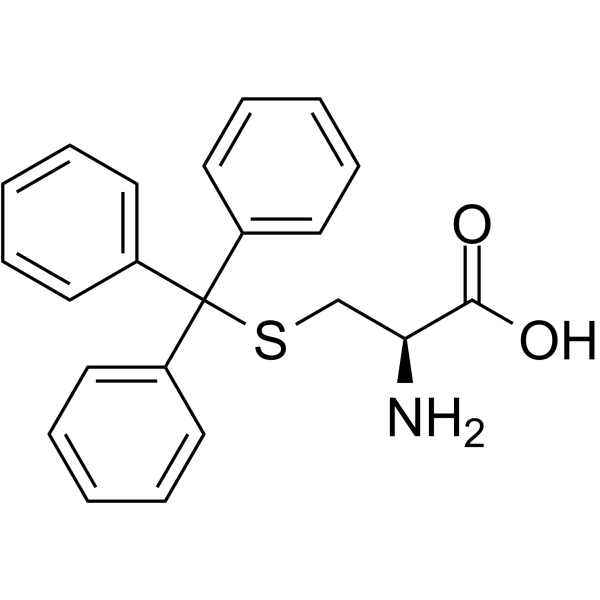
S-Tritylcysteine structure
|
Common Name | S-Tritylcysteine | ||
|---|---|---|---|---|
| CAS Number | 2799-07-7 | Molecular Weight | 363.47 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 524.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H21NO2S | Melting Point | 182-183 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 271.2±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of S-TritylcysteineS-Trityl-L-cysteine (NSC 83265) is a selective and allosteric kinesin Eg5 inhibitor with an IC50 of 1 μM for the inhibition of basal ATPase activity and 140 nM for the microtubule-activated ATPase activity. S-Trityl-L-cysteine has antitumor activities[1][2]. |
| Name | S-Trityl-L-cysteine |
|---|---|
| Synonym | More Synonyms |
| Description | S-Trityl-L-cysteine (NSC 83265) is a selective and allosteric kinesin Eg5 inhibitor with an IC50 of 1 μM for the inhibition of basal ATPase activity and 140 nM for the microtubule-activated ATPase activity. S-Trityl-L-cysteine has antitumor activities[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Eg5 |
| In Vitro | S-Trityl-L-cysteine binds to a unique pocket in the Eg5 motor domain formed by secondary structural elements (helix a2/loop L5/helix a3)[1]. S-Trityl-L-cysteine (1-20 µM; for 72 h) could mediate cell apoptosis, as well as cell cycle arrest, in a dose-dependent manner. S-Trityl-L-cysteine-mediated apoptosis and cell cycle arrest were triggered by activation of the mitogen-activated protein kinase and nuclear factor kB signaling pathways[1]. S-Trityl-L-cysteine induces mitotic arrest in HeLa cells (IC50 of 700 nM) with characteristic monoastral spindles[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 524.7±50.0 °C at 760 mmHg |
| Melting Point | 182-183 °C (dec.)(lit.) |
| Molecular Formula | C22H21NO2S |
| Molecular Weight | 363.47 |
| Flash Point | 271.2±30.1 °C |
| Exact Mass | 363.129303 |
| PSA | 88.62000 |
| LogP | 5.56 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.642 |
| InChIKey | DLMYFMLKORXJPO-FQEVSTJZSA-N |
| SMILES | NC(CSC(c1ccccc1)(c1ccccc1)c1ccccc1)C(=O)O |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AY7710000 |
| HS Code | 2930909090 |
|
~98% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: Silvia, Vertuani; Baldisserotto, Anna; Scalambra, Emanuela; Malisardi, Gemma; Durini, Elisa; Manfredini, Stefano European Journal of Medicinal Chemistry, 2012 , vol. 50, p. 383 - 392 |
|
~89% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: Marquette University; University of Wisconsin - Milwaukee Patent: US7829709 B1, 2010 ; Location in patent: Page/Page column 19-20 ; |
|
~90% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: Jin, Hui-Juan; Lu, Jing; Wu, Xue Bioorganic and Medicinal Chemistry, 2012 , vol. 20, # 11 p. 3465 - 3469 |
|
~83% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: Chen, Chun-Chi; Rajagopal, Basker; Liu, Xuan Yu; Chen, Kuan Lin; Tyan, Yu-Chang; Lin, Fui; Lin, Po-Chiao Amino Acids, 2014 , vol. 46, # 2 p. 367 - 374 |
|
~% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: WO2007/9944 A1, ; Page/Page column 31-32 ; |
|
~% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: WO2006/102243 A2, ; Page/Page column 63 ; WO 2006/102243 A2 |
|
~% 
S-Tritylcysteine CAS#:2799-07-7 |
| Literature: Bulletin de la Societe Chimique de France, , p. 698 |
| Precursor 6 | |
|---|---|
| DownStream 8 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Cdk1 phosphorylates the Rac activator Tiam1 to activate centrosomal Pak and promote mitotic spindle formation.
Nat. Commun. 6 , 7437, (2015) Centrosome separation is critical for bipolar spindle formation and the accurate segregation of chromosomes during mammalian cell mitosis. Kinesin-5 (Eg5) is a microtubule motor essential for centroso... |
|
|
Structure-activity relationship of S-trityl-L-cysteine analogues as inhibitors of the human mitotic kinesin Eg5.
J. Med. Chem. 51 , 1115-25, (2008) The human kinesin Eg5 is a potential drug target for cancer chemotherapy. Eg5 specific inhibitors cause cells to block in mitosis with a characteristic monoastral spindle phenotype. Prolonged metaphas... |
|
|
Dynamics of Human Telomerase Holoenzyme Assembly and Subunit Exchange across the Cell Cycle.
J. Biol. Chem. 290 , 21320-35, (2015) Human telomerase acts on telomeres during the genome synthesis phase of the cell cycle, accompanied by its concentration in Cajal bodies and transient colocalization with telomeres. Whether the regula... |
| S-Triphenylmethyl-L-cysteine |
| EG5 INHIBITOR II |
| Cys(Trt)-OH |
| 3-tritylthio-L-alanine |
| Alanine, 3- (tritylthio)-, D- |
| L-Cys(trt)-OH |
| DL-Cysteine, S-(triphenylmethyl)- |
| L-CYSTEINE, S-(TRIPHENYLMETHYL)- |
| EINECS 218-195-8 |
| S-Tritylcysteine |
| (R)-S-Tritylcysteine |
| (2S)-2-Ammonio-3-(tritylsulfanyl)propanoate |
| Cysteine, S-(triphenylmethyl)- |
| D-Cysteine, S- (triphenylmethyl)- |
| S-Trt-L-Lys-OH |
| H-Cys(Trt)-OH |
| L-Cysteine, S- (triphenylmethyl)- |
| TRITYL-L-CYSTEINE |
| H-L-Cys(Trt)-OH |
| MFCD00002611 |
| CYSTEINE(TRT)-OH |
| Alanine, 3- (tritylthio)-, L- |
| Tritylthioalanine |
| Ethanaminium, 1-carboxy-2-[(triphenylmethyl)thio]-, inner salt, (1S)- |

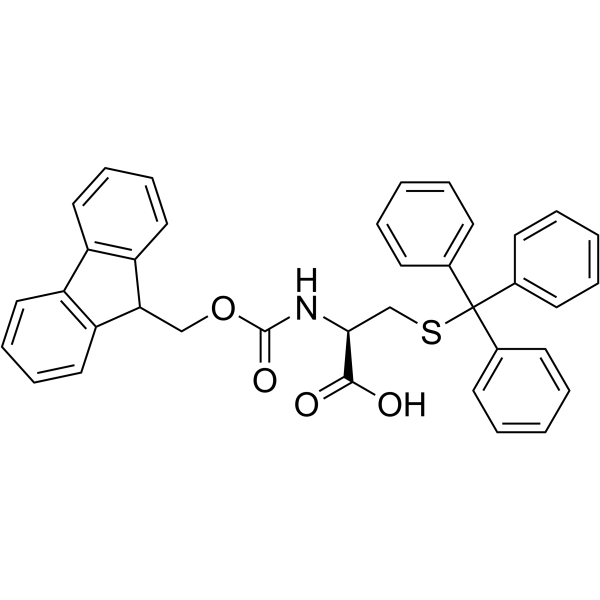
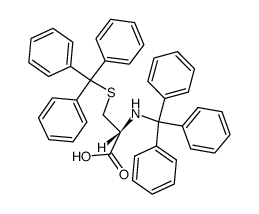
 CAS#:519-73-3
CAS#:519-73-3![L-Cysteine,N-[(phenylmethoxy)carbonyl]-S-(triphenylmethyl)- structure](https://image.chemsrc.com/caspic/159/26311-04-6.png) CAS#:26311-04-6
CAS#:26311-04-6 CAS#:21947-98-8
CAS#:21947-98-8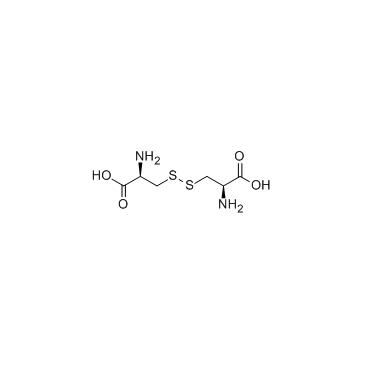 CAS#:56-89-3
CAS#:56-89-3![Glycine,N-[N-[(phenylmethoxy)carbonyl]-S-(triphenylmethyl)-L-cysteinyl]-, ethyl ester(9CI) structure](https://image.chemsrc.com/caspic/159/3695-78-1.png) CAS#:3695-78-1
CAS#:3695-78-1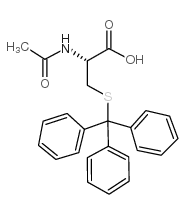 CAS#:27486-87-9
CAS#:27486-87-9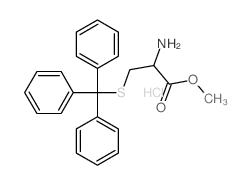 CAS#:62675-68-7
CAS#:62675-68-7
