Bioorganic & Medicinal Chemistry
2009-08-15
Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors.
Peng Zhan, Xinyong Liu, Junjie Zhu, Zengjun Fang, Zhenyu Li, Christophe Pannecouque, Erik De Clercq
Index: Bioorg. Med. Chem. 17(16) , 5775-81, (2009)
Full Text: HTML
Abstract
A series of 2-(1-aryl-1H-imidazol-2-ylthio)acetamide [imidazole thioacetanilide (ITA)] derivatives were synthesized and evaluated as potent inhibitors of human immunodeficiency virus type-1 (HIV-1). Among them, the most potent HIV-1 inhibitors were 4a5 (EC(50)=0.18microM), and 4a2 (EC(50)=0.20microM), which were more effective than the lead compound L1 (EC(50)=2.053microM) and the reference drugs nevirapine and delavirdine. The preliminary structure-activity relationship (SAR) of the newly synthesized congeners is discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
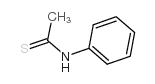 |
Thioacetanilide
CAS:637-53-6 |
C8H9NS |
Related Articles:
More...
|
The metabolism of thioacetanilide in the rat.
1983-08-01 [Xenobiotica 13(8) , 475-82, (1983)] |
|
1,2,3-Selenadiazole thioacetanilides: synthesis and anti-HIV...
2009-09-01 [Bioorg. Med. Chem. 17(17) , 6374-9, (2009)] |
|
Effects from filtration, capping agents, and presence/absenc...
2010-12-01 [Bioorg. Med. Chem. 20(18) , 5527-36, (2012)] |
|
[Substantiation of maximum permissible levels of thioacylani...
1986-07-01 [Gig. Tr. Prof. Zabol. (7) , 51-2, (1986)] |
|
Application of beta-(2-chloroaroyl) thioacetanilides in synt...
2008-03-07 [J. Org. Chem. 73(5) , 1852-63, (2008)] |