Application of beta-(2-chloroaroyl) thioacetanilides in synthesis: an unusual and highly efficient access to thiochromeno[2,3-b]pyridine derivatives.
Li-Rong Wen, Ji-Hui Sun, Ming Li, En-Tao Sun, Shu-Sheng Zhang
Index: J. Org. Chem. 73(5) , 1852-63, (2008)
Full Text: HTML
Abstract
A series of unusual fused tricyclic thiochromeno[2,3-b]pyridines were successfully synthesized by tandem [3 + 3] annulation and SNAr of beta-(2-chloroaroyl) thioacetanilides with activated 4-arylidene-2-phenyloxazol-5(4H)-ones or aromatic aldehydes and ethyl 2-cyanoacetate under microwave irradiation, respectively. Because of the existence of the o-chloro atom of beta-(2-chloroaroyl) thioacetanilides, these reactions were very mild, convenient, and ortho-selective to form new fused tricyclic target molecules. In the domino processes, at least seven reactive distinct chemical sites were involved and up to three new covalent bonds and one tricycle with only cis configuration were generated. A synthetic study and mechanistic proposal for these transformations are presented.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
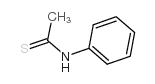 |
Thioacetanilide
CAS:637-53-6 |
C8H9NS |
|
The metabolism of thioacetanilide in the rat.
1983-08-01 [Xenobiotica 13(8) , 475-82, (1983)] |
|
1,2,3-Selenadiazole thioacetanilides: synthesis and anti-HIV...
2009-09-01 [Bioorg. Med. Chem. 17(17) , 6374-9, (2009)] |
|
Synthesis and biological evaluation of imidazole thioacetani...
2009-08-15 [Bioorg. Med. Chem. 17(16) , 5775-81, (2009)] |
|
Effects from filtration, capping agents, and presence/absenc...
2010-12-01 [Bioorg. Med. Chem. 20(18) , 5527-36, (2012)] |
|
[Substantiation of maximum permissible levels of thioacylani...
1986-07-01 [Gig. Tr. Prof. Zabol. (7) , 51-2, (1986)] |