Removal of hydrogen chloride from gaseous streams using magnesium-aluminum oxide.
Tomohito Kameda, Naoya Uchiyama, Kye-Sung Park, Guido Grause, Toshiaki Yoshioka
Index: Chemosphere 73(5) , 844-7, (2008)
Full Text: HTML
Abstract
Magnesium-aluminum oxide (Mg-Al oxide) obtained by thermal decomposition of Mg-Al layered double hydroxide (Mg-Al LDH) effectively removed HCl from gaseous streams. HCl removal was greater in the presence of added water vapor at all temperatures examined and increased with decreasing temperature in both the presence and absence of added water vapor. Wet and dry removal of gaseous HCl were attributed to the production of MgCl2 . 6H2O and MgCl2 . 4H2O, respectively. For the wet scrubbing process, the reconstruction reaction of Mg-Al LDH from Mg-Al oxide was the primary mechanism for increased HCl removal.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
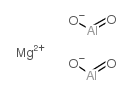 |
magnesium aluminate
CAS:12068-51-8 |
Al2H2MgO4 |
|
Epitaxially driven formation of intricate supported gold nan...
2009-12-01 [Nano Lett. 9(12) , 4258-63, (2009)] |
|
Removal of tetrafluoroborate ion from aqueous solution using...
2007-10-01 [Chemosphere 69(5) , 832-5, (2007)] |
|
Removal of HCl, SO₂, and NO by treatment of acid gas with Mg...
2011-01-01 [Chemosphere 82(4) , 587-91, (2011)] |
|
Study on adsorption of glyphosate (N-phosphonomethyl glycine...
2005-10-17 [J. Hazard. Mater. 125(1-3) , 89-95, (2005)] |
|
Spectrophotometric evaluation of the optical influence of co...
2009-01-01 [Dent. Mater. 25(2) , 158-65, (2009)] |