magnesium aluminate
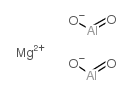
magnesium aluminate structure
|
Common Name | magnesium aluminate | ||
|---|---|---|---|---|
| CAS Number | 12068-51-8 | Molecular Weight | 144.28200 | |
| Density | 3.64 g/mL at 25 °C(lit.) | Boiling Point | N/A | |
| Molecular Formula | Al2H2MgO4 | Melting Point | 2130 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Epitaxially driven formation of intricate supported gold nanostructures on a lattice-matched oxide substrate.
Nano Lett. 9(12) , 4258-63, (2009) A new class of gold nanostructures has been fabricated on the (100), (111), and (110) surfaces of lattice-matched MgAl(2)O(4) substrates. The nanostructures were fabricated through a synthesis route where a thin gold film dewets, liquefies, and then slowly se... |
|
|
Removal of tetrafluoroborate ion from aqueous solution using magnesium-aluminum oxide produced by the thermal decomposition of a hydrotalcite-like compound.
Chemosphere 69(5) , 832-5, (2007) Magnesium-aluminum oxide (Mg-Al oxide) prepared by the thermal decomposition of a hydrotalcite-like compound was found to have potential for treating NaBF(4) wastewater. The Mg-Al oxide removed the BF(4)(-) and F(-) and H(3)BO(3) from the NaBF(4) solution. Wi... |
|
|
Removal of HCl, SO₂, and NO by treatment of acid gas with Mg-Al oxide slurry.
Chemosphere 82(4) , 587-91, (2011) Although effective treatment of acid gases such as HCl, SO(x), and NO(x) is essential for preventing air pollution, current methods pose other environmental problems such as CaCl₂ leaching, reduced landfill lifetimes, and solid waste production. Here we show ... |
|
|
Study on adsorption of glyphosate (N-phosphonomethyl glycine) pesticide on MgAl-layered double hydroxides in aqueous solution.
J. Hazard. Mater. 125(1-3) , 89-95, (2005) MgAl-layered double hydroxides with different interlayer anions (nitrate, carbonate and chloride) were evaluated for their abilities to adsorb the organic pesticide glyphosate (N-phosphonomethyl glycine, Gly). The adsorption isotherms of Gly on layered double... |
|
|
Spectrophotometric evaluation of the optical influence of core build-up composites on all-ceramic materials
Dent. Mater. 25(2) , 158-65, (2009) Objectives To evaluate the optical influence of core build-up composites on the resultant color of ceramic–composite combinations, and analyze the color difference of ceramic–composite combinations to Vita Lumin shade guide with the same nominal shade. |
|
|
Removal of hydrogen chloride from gaseous streams using magnesium-aluminum oxide.
Chemosphere 73(5) , 844-7, (2008) Magnesium-aluminum oxide (Mg-Al oxide) obtained by thermal decomposition of Mg-Al layered double hydroxide (Mg-Al LDH) effectively removed HCl from gaseous streams. HCl removal was greater in the presence of added water vapor at all temperatures examined and ... |
|
|
Bromide ion removal from contaminated water by calcined and uncalcined MgAl-CO3 layered double hydroxides.
J. Hazard. Mater. 152(3) , 1130-7, (2008) A fundamental investigation on the uptake of bromide ion from contaminated water by calcined and uncalcined MgAl-CO3 layered double hydroxides (LDHs) were conducted in batch mode. The uptake capacity of calcined LDHs (CLDH) is higher than that of uncalcined L... |
|
|
In vitro attachment of osteoblast-like cells to osteoceramic materials.
Dent. Mater. 13(1) , 62-8, (1997) The objective of this work was to examine osteoblast-like cell attachment and morphology in vitro to osteoceramic materials with three different surface morphologies.Osteoceramic composite disks were fabricated from tricalcium phosphate and magnesium-aluminat... |
|
|
Molecular interaction in alginate beads reinforced with sodium starch glycolate or magnesium aluminum silicate, and their physical characteristics.
Int. J. Pharm. 293(1-2) , 51-62, (2005) Diclofenac calcium-alginate (DCA) beads were reinforced with different amounts of sodium starch glycolate (SSG) or magnesium aluminum silicate (MAS) and were prepared using ionotropic gelation method. Complex formation of sodium alginate (SA) and SSG or MAS i... |
|
|
Treatment of waste H₂SO₄ with Mg-Al oxide obtained by calcination of NO₃⁻-intercalated Mg-Al layered double hydroxide: Kinetics and equilibrium.
J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 47(5) , 711-7, (2012) Mg-Al oxide obtained by calcination of NO(3)(-)-intercalated Mg-Al layered double hydroxide (NO(3)•Mg-Al LDH) was used to treat H(2)SO(4), acting as both a neutralizer of the acid and a fixative for SO(4)(2-). The fraction of SO(4)(2-) removed increased with ... |