Molecular interaction in alginate beads reinforced with sodium starch glycolate or magnesium aluminum silicate, and their physical characteristics.
Satit Puttipipatkhachorn, Thaned Pongjanyakul, Aroonsri Priprem
Index: Int. J. Pharm. 293(1-2) , 51-62, (2005)
Full Text: HTML
Abstract
Diclofenac calcium-alginate (DCA) beads were reinforced with different amounts of sodium starch glycolate (SSG) or magnesium aluminum silicate (MAS) and were prepared using ionotropic gelation method. Complex formation of sodium alginate (SA) and SSG or MAS in calcium-alginate beads was revealed using FTIR spectroscopy. Differential scanning calorimetric study indicated that diclofenac sodium (DS) in amorphous form was dispersed in the matrix of DCA beads. The thermal behavior of SSG-DCA and MAS-DCA beads was similar to the control bead. Both additives can improve the entrapment efficiency of DCA beads. The swelling and water uptake of the beads depended on the properties of incorporated additives. The SSG-DCA beads showed a higher water uptake and swelling than MAS-DCA beads. Moreover, the swelling of the beads showed a good correlation with the square root of time. The release kinetic of the beads in pH 6.8 phosphate buffer was swelling controlled mechanism, while that in distilled water followed Higuchi's model. The slower release rate and the longer lag time in pH 6.8 phosphate buffer was obtained from the SSG-DCA and MAS-DCA beads because of complex formation between SA and SSG or MAS. However, SSG in the beads could increase the release of DS from the beads in distilled water because it acted as a channeling agent. In contrast, MAS retarded the release of DS from the beads in distilled water due to the stronger matrix formation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
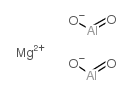 |
magnesium aluminate
CAS:12068-51-8 |
Al2H2MgO4 |
|
Epitaxially driven formation of intricate supported gold nan...
2009-12-01 [Nano Lett. 9(12) , 4258-63, (2009)] |
|
Removal of tetrafluoroborate ion from aqueous solution using...
2007-10-01 [Chemosphere 69(5) , 832-5, (2007)] |
|
Removal of HCl, SO₂, and NO by treatment of acid gas with Mg...
2011-01-01 [Chemosphere 82(4) , 587-91, (2011)] |
|
Study on adsorption of glyphosate (N-phosphonomethyl glycine...
2005-10-17 [J. Hazard. Mater. 125(1-3) , 89-95, (2005)] |
|
Spectrophotometric evaluation of the optical influence of co...
2009-01-01 [Dent. Mater. 25(2) , 158-65, (2009)] |