Treatment of waste H₂SO₄ with Mg-Al oxide obtained by calcination of NO₃⁻-intercalated Mg-Al layered double hydroxide: Kinetics and equilibrium.
Tomohito Kameda, Yuki Fubasami, Toshiaki Yoshioka
Index: J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 47(5) , 711-7, (2012)
Full Text: HTML
Abstract
Mg-Al oxide obtained by calcination of NO(3)(-)-intercalated Mg-Al layered double hydroxide (NO(3)•Mg-Al LDH) was used to treat H(2)SO(4), acting as both a neutralizer of the acid and a fixative for SO(4)(2-). The fraction of SO(4)(2-) removed increased with time and with increasing Mg-Al oxide quantity and temperature. The rate of SO(4)(2-) removal followed first-order kinetics with apparent rate constants of 2.0 × 10(-3), 4.4 × 10(-3), and 5.3 × 10(-2) min(-1) at 10, 30, and 60°C, respectively. The apparent activation energy was 52.1 kJ mol(-1), confirming that the SO(4)(2-) removal by Mg-Al oxide proceeded under chemical reaction control. Furthermore, the adsorption isotherm of SO(4)(2-) by Mg-Al oxide obeyed the Langmuir equation. The maximum adsorption amount was 2.0 mmol g(-1), or 4.0 meq g(-1), indicating that Mg-Al oxide has a large capacity for uptake of SO(4)(2-) from H(2)SO(4).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
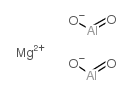 |
magnesium aluminate
CAS:12068-51-8 |
Al2H2MgO4 |
|
Epitaxially driven formation of intricate supported gold nan...
2009-12-01 [Nano Lett. 9(12) , 4258-63, (2009)] |
|
Removal of tetrafluoroborate ion from aqueous solution using...
2007-10-01 [Chemosphere 69(5) , 832-5, (2007)] |
|
Removal of HCl, SO₂, and NO by treatment of acid gas with Mg...
2011-01-01 [Chemosphere 82(4) , 587-91, (2011)] |
|
Study on adsorption of glyphosate (N-phosphonomethyl glycine...
2005-10-17 [J. Hazard. Mater. 125(1-3) , 89-95, (2005)] |
|
Spectrophotometric evaluation of the optical influence of co...
2009-01-01 [Dent. Mater. 25(2) , 158-65, (2009)] |