Etiocholanolone
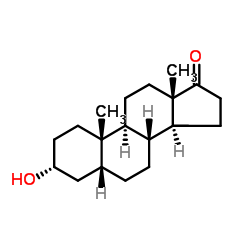
Etiocholanolone structure
|
Common Name | Etiocholanolone | ||
|---|---|---|---|---|
| CAS Number | 53-42-9 | Molecular Weight | 290.440 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H30O2 | Melting Point | 148~150°C (lit.) | |
| MSDS | Chinese USA | Flash Point | 176.4±21.3 °C | |
|
An updated steroid benchmark set and its application in the discovery of novel nanomolar ligands of sex hormone-binding globulin.
J. Med. Chem. 51 , 2047-56, (2008) A benchmark data set of steroids with known affinity for sex hormone-binding globulin (SHBG) has been widely used to validate popular molecular field-based QSAR techniques. We have expanded the data set by adding a number of nonsteroidal SHBG ligands identifi... |
|
|
Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies.
J. Med. Chem. 51 , 1831-41, (2008) TGR5, a metabotropic receptor that is G-protein-coupled to the induction of adenylate cyclase, has been recognized as the molecular link connecting bile acids to the control of energy and glucose homeostasis. With the aim of disclosing novel selective modulat... |
|
|
Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens.
Eur. J. Med. Chem. 43 , 107-13, (2008) Allopregnanolone (1) and pregnanolone (2), steroids containing a 17beta-acetyl group, are potent enhancers of GABA (gamma-aminobutyric acid) action at GABAA receptors. Their effects are enantioselective with the non-naturally occurring enantiomers (ent-1 and ... |
|
|
Neurosteroid analogues. 11. Alternative ring system scaffolds: gamma-aminobutyric acid receptor modulation and anesthetic actions of benz[f]indenes.
J. Med. Chem. 49 , 4595-605, (2006) Benz[f]indenes are tricyclic compounds with a linear 6-6-5 fused carbocyclic ring system. When properly substituted, benz[f]indenes can satisfy the pharmacophore requirements of the critical hydrogen-bond donor and acceptor groups found in neuroactive steroid... |
|
|
Release of free and conjugated forms of the putative pheromonal steroid 11-oxo-etiocholanolone by reproductively mature male round goby (Neogobius melanostomus Pallas, 1814).
Biol. Reprod. 84 , 288-98, (2011) Previous studies of the round goby (Neogobius melanostomus Pallas, 1814), an invasive fish species in the Laurentian Great Lakes of North America, have shown that this species has the ability to both synthesize and smell steroids that have a 5 beta-reduced an... |
|
|
Urinary steroid profile in early pregnancy after in vitro fertilization.
Acta Obstet. Gynecol. Scand. 91(5) , 625-9, (2012) The aim of the study was to compare the levels of urinary steroid metabolites of patients with successful in vitro fertilization and patients who failed to achieve pregnancy.Comparison of urinary steroid profiles prior to oocyte pick-up and three weeks after ... |
|
|
Development of a GC/C/IRMS method--confirmation of a novel steroid profiling approach in doping control.
Steroids 77(11) , 1050-60, (2012) In doping control, an athlete can only be convicted with the misuse with endogenous steroids like testosterone (T), if abnormal values of steroid metabolites and steroid ratios are observed and if the subsequent analysis with isotope ratios mass spectrometry ... |
|
|
Certification of steroid carbon isotope ratios in a freeze-dried human urine reference material.
Drug Test. Anal. 4(12) , 928-33, (2012) An accurate method for the measurement of carbon isotope ratios of steroids in human urine has been developed at the National Measurement Institute, Australia (NMIA) for the certification of a freeze-dried human urine reference material (CRM NMIA MX005). The ... |
|
|
Urine stability and steroid profile: towards a screening index of urine sample degradation for anti-doping purpose.
Anal. Chim. Acta 683(2) , 221-6, (2011) The presence of microorganisms in urine samples, under favourable conditions of storage and transportation, may alter the concentration of steroid hormones, thus altering the correct evaluation of the urinary steroid profile in doping control analysis. Accord... |
|
|
Detection of synthetic testosterone use by novel comprehensive two-dimensional gas chromatography combustion-isotope ratio mass spectrometry.
Anal. Chem. 83(18) , 7158-65, (2011) We report the first demonstration of comprehensive two-dimensional gas chromatography combustion-isotope ratio mass spectrometry (GC×GCC-IRMS) for the analysis of urinary steroids to detect illicit synthetic testosterone use, of interest in sport doping. GC c... |