Etiocholanolone
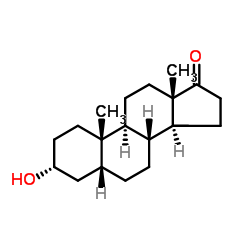
Etiocholanolone structure
|
Common Name | Etiocholanolone | ||
|---|---|---|---|---|
| CAS Number | 53-42-9 | Molecular Weight | 290.440 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H30O2 | Melting Point | 148~150°C (lit.) | |
| MSDS | Chinese USA | Flash Point | 176.4±21.3 °C | |
Use of EtiocholanoloneEtiocholanolone (5β-Androsterone) is the excreted metabolite of testosterone and has anticonvulsant activity[1]. Etiocholanolone is a less potent neurosteroid positive allosteric modulator (PAM) of the GABAA receptor than its enantiomer form[2]. |
| Name | 3α-hydroxy-5β-androstan-17-one |
|---|---|
| Synonym | More Synonyms |
| Description | Etiocholanolone (5β-Androsterone) is the excreted metabolite of testosterone and has anticonvulsant activity[1]. Etiocholanolone is a less potent neurosteroid positive allosteric modulator (PAM) of the GABAA receptor than its enantiomer form[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Etiocholanolone (10 μM) coapplication with GABA leads to an increase in the relative frequency of long openings (fraction of OT3, site A2 effect), but it is ineffective at increasing the duration of long openings (site B effect) or at decreasing the relative frequency of the activation-related closed time component (site A1 effect)[2]. |
| In Vivo | Etiocholanolone (intraperitoneal injection; 0-109.1 mg/kg; single dose) exhibits ED50 values of 57.6 and 109.1 mg/kg in the 6-Hz and PTZ tests, respectively. Protective activity in the 6-Hz test of 5β,3α-A persists for 2 h and is shorter than ent-5β,3α-A treatment (3 hours) in mice[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±45.0 °C at 760 mmHg |
| Melting Point | 148~150°C (lit.) |
| Molecular Formula | C19H30O2 |
| Molecular Weight | 290.440 |
| Flash Point | 176.4±21.3 °C |
| Exact Mass | 290.224579 |
| PSA | 37.30000 |
| LogP | 3.75 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | QGXBDMJGAMFCBF-BNSUEQOYSA-N |
| SMILES | CC12CCC3C(CCC4CC(O)CCC43C)C1CCC2=O |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F,Xn |
| Risk Phrases | 11-20/21/22-36 |
| Safety Phrases | 16-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
|
An updated steroid benchmark set and its application in the discovery of novel nanomolar ligands of sex hormone-binding globulin.
J. Med. Chem. 51 , 2047-56, (2008) A benchmark data set of steroids with known affinity for sex hormone-binding globulin (SHBG) has been widely used to validate popular molecular field-based QSAR techniques. We have expanded the data s... |
|
|
Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies.
J. Med. Chem. 51 , 1831-41, (2008) TGR5, a metabotropic receptor that is G-protein-coupled to the induction of adenylate cyclase, has been recognized as the molecular link connecting bile acids to the control of energy and glucose home... |
|
|
Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens.
Eur. J. Med. Chem. 43 , 107-13, (2008) Allopregnanolone (1) and pregnanolone (2), steroids containing a 17beta-acetyl group, are potent enhancers of GABA (gamma-aminobutyric acid) action at GABAA receptors. Their effects are enantioselecti... |
| 3a-Hydroxy-5b-androstane-17-one |
| Androsterone, (5β)- |
| 3alpha-hydroxy-5beta-androstan-17-one |
| 5β-Androsterone |
| (5b)-Androsterone |
| 5-Isoandrosterone |
| 3α-hydroxy-5β-androstane-17-one |
| 5β-Androstan-17-one, 3α-hydroxy- |
| 5BETA-ANDROSTERONE |
| MFCD00064133 |
| (3α,5β)-3-Hydroxyandrostan-17-one |
| ETIOCHOLANOLON |
| 3α-Etiocholanolone |
| Androstan-17-one, 3-hydroxy-, (3α,5β)- |
| 19-noretiocholanolone |
| ETIOCHOLANONE |
| 3a-Etiocholanolone |
| 3α-hydroxy-5β-androstan-17-one |
| 5b-Androsterone |
| Etiocholan-3a-ol-17-one |
| Etiocholan-3α-ol-17-one |
| Etiocholan-3.α.-ol-17-one |
| EINECS 200-835-2 |
| Etiocholanolone |
 CAS#:1229-12-5
CAS#:1229-12-5 CAS#:1044-89-9
CAS#:1044-89-9 CAS#:571-20-0
CAS#:571-20-0 CAS#:63-05-8
CAS#:63-05-8 CAS#:68-60-0
CAS#:68-60-0 CAS#:566-42-7
CAS#:566-42-7 CAS#:26439-43-0
CAS#:26439-43-0 CAS#:128-20-1
CAS#:128-20-1 CAS#:4062-43-5
CAS#:4062-43-5 CAS#:80-92-2
CAS#:80-92-2