| Structure | Name/CAS No. | Articles |
|---|---|---|
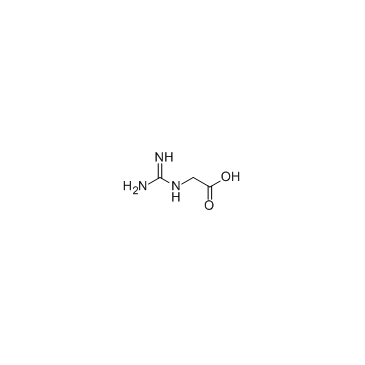 |
2-Guanidinoacetic acid
CAS:352-97-6 |
|
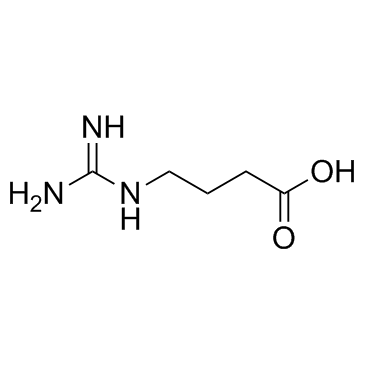 |
4-Guanidinobutanoic acid
CAS:463-00-3 |
|
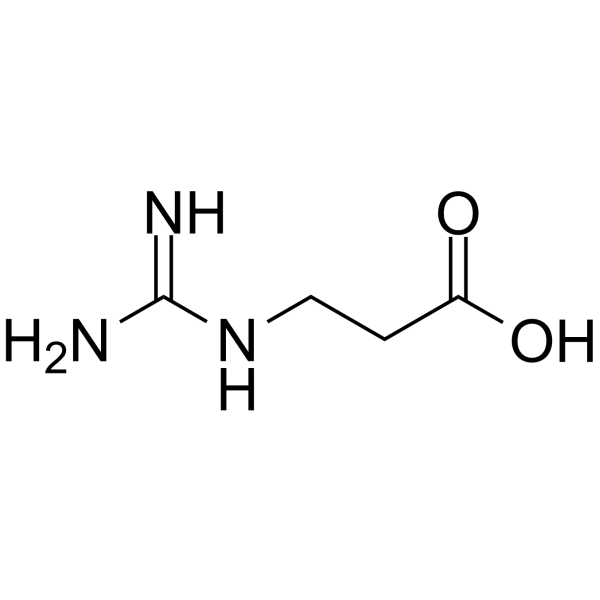 |
RGX-202
CAS:353-09-3 |
|
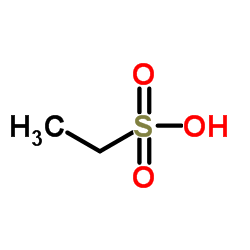 |
Ethanesulfonic acid
CAS:594-45-6 |
|
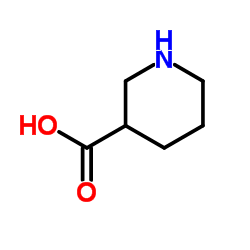 |
Nipecotic acid
CAS:498-95-3 |
|
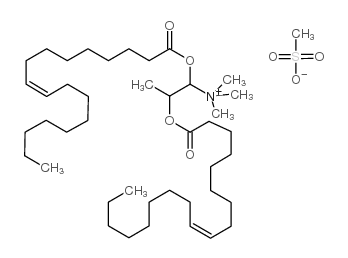 |
DOTAP Transfection Reagent
CAS:144189-73-1 |
|
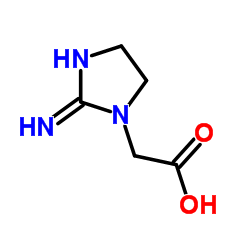 |
(2-Iminoimidazolidin-1-yl)acetic acid
CAS:35404-50-3 |