Journal of Organic Chemistry
2013-02-01
Cycloaromatization approach to polysubstituted indolizines from 2-acetylpyrroles: decoration of the pyridine unit.
Jin Ho Lee, Ikyon Kim
Index: J. Org. Chem. 78(3) , 1283-8, (2013)
Full Text: HTML
Abstract
A new synthetic route to indolizines with various substituents on the pyridine moiety was developed by utilizing a facile cycloaromatization of 2-acetylpyrrole derivatives. Without isolation, the resulting intermediates were allowed to react with various electrophiles to afford a range of indolizines. In particular, Suzuki-Miyaura cross-coupling of O-triflates with (hetero)arylboronic acids permitted introduction of diverse substituents at the C8 position of an indolizine skeleton.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
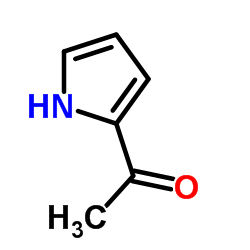 |
2-Acetyl-1H-pyrrole
CAS:1072-83-9 |
C6H7NO |
Related Articles:
More...
|
Approaches of aroma extraction dilution analysis (AEDA) for ...
2015-11-15 [Food Chem. 187 , 44-52, (2015)] |
|
Extension of a dynamic headspace multi-volatile method to mi...
2015-11-20 [J. Chromatogr. A. 1421 , 103-13, (2015)] |
|
Mutagen formation in the reaction of Maillard browning produ...
1986-12-01 [Food Chem. Toxicol. 24(12) , 1303-8, (1986)] |
|
2- and 3-acetylpyrroles: a combined calorimetric and computa...
2009-04-16 [J. Phys. Chem. A 113(15) , 3630-8, (2009)] |
|
Pyrrole alkaloids from Bolbostemma paniculatum.
2003-09-01 [J. Asian Nat. Prod. Res. 5(3) , 159-63, (2003)] |