2- and 3-acetylpyrroles: a combined calorimetric and computational study.
Ana Filipa L O M Santos, José R B Gomes, Manuel A V Ribeiro da Silva
Index: J. Phys. Chem. A 113(15) , 3630-8, (2009)
Full Text: HTML
Abstract
A combined experimental and computational study on the thermochemistry of 2- and 3-acetylpyrroles was performed. The enthalpies of combustion and sublimation were measured by static bomb combustion calorimetry and Knudsen effusion mass-loss technique, respectively, and the standard (p(o) = 0.1 MPa) molar enthalpies of formation, in the gaseous phase, at T = 298.15 K, were determined. Additionally, the gas-phase enthalpies of formation were estimated by G3(MP2)//B3LYP calculations, using several gas-phase working reactions, and were compared with the experimental ones. N-H bond dissociation enthalpies, gas-phase acidities and basicities, proton and electron affinities and ionization enthalpies were also calculated. Experimental and theoretical results are in good agreement and show that 2-acetylpyrrole is thermodynamically more stable than the 3-isomer. The substituent effects of the acetyl group in pyrrole, thiophene and pyridine rings were also analyzed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
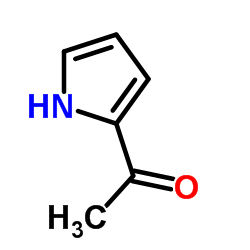 |
2-Acetyl-1H-pyrrole
CAS:1072-83-9 |
C6H7NO |
|
Approaches of aroma extraction dilution analysis (AEDA) for ...
2015-11-15 [Food Chem. 187 , 44-52, (2015)] |
|
Extension of a dynamic headspace multi-volatile method to mi...
2015-11-20 [J. Chromatogr. A. 1421 , 103-13, (2015)] |
|
Mutagen formation in the reaction of Maillard browning produ...
1986-12-01 [Food Chem. Toxicol. 24(12) , 1303-8, (1986)] |
|
Cycloaromatization approach to polysubstituted indolizines f...
2013-02-01 [J. Org. Chem. 78(3) , 1283-8, (2013)] |
|
Pyrrole alkaloids from Bolbostemma paniculatum.
2003-09-01 [J. Asian Nat. Prod. Res. 5(3) , 159-63, (2003)] |