2-Acetyl-1H-pyrrole
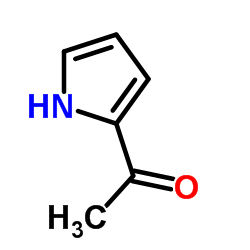
2-Acetyl-1H-pyrrole structure
|
Common Name | 2-Acetyl-1H-pyrrole | ||
|---|---|---|---|---|
| CAS Number | 1072-83-9 | Molecular Weight | 109.126 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 220.9±13.0 °C at 760 mmHg | |
| Molecular Formula | C6H7NO | Melting Point | 88-93 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 92.4±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Approaches of aroma extraction dilution analysis (AEDA) for headspace solid phase microextraction and gas chromatography-olfactometry (HS-SPME-GC-O): Altering sample amount, diluting the sample or adjusting split ratio?
Food Chem. 187 , 44-52, (2015) Aroma extract dilution analysis (AEDA) is widely used for the screening of aroma-active compounds in gas chromatography-olfactometry (GC-O). In this study, three aroma dilution methods, (I) using different test sample volumes, (II) diluting samples, and (III)... |
|
|
Extension of a dynamic headspace multi-volatile method to milliliter injection volumes with full sample evaporation: Application to green tea.
J. Chromatogr. A. 1421 , 103-13, (2015) An extension of multi-volatile method (MVM) technology using the combination of a standard dynamic headspace (DHS) configuration, and a modified DHS configuration incorporating an additional vacuum module, was developed for milliliter injection volume of aque... |
|
|
Mutagen formation in the reaction of Maillard browning products, 2-acetylpyrrole and its analogues, with nitrite.
Food Chem. Toxicol. 24(12) , 1303-8, (1986) Three 2-substituted pyrroles (2-acetylpyrrole, pyrrole-2-carboxaldehyde and pyrrole-2-carboxylic acid), which are products of the Maillard browning reaction, were reacted with nitrite in buffer solution (pH 3) at 50 degrees C for 24 hr. The reaction mixtures ... |
|
|
Cycloaromatization approach to polysubstituted indolizines from 2-acetylpyrroles: decoration of the pyridine unit.
J. Org. Chem. 78(3) , 1283-8, (2013) A new synthetic route to indolizines with various substituents on the pyridine moiety was developed by utilizing a facile cycloaromatization of 2-acetylpyrrole derivatives. Without isolation, the resulting intermediates were allowed to react with various elec... |
|
|
2- and 3-acetylpyrroles: a combined calorimetric and computational study.
J. Phys. Chem. A 113(15) , 3630-8, (2009) A combined experimental and computational study on the thermochemistry of 2- and 3-acetylpyrroles was performed. The enthalpies of combustion and sublimation were measured by static bomb combustion calorimetry and Knudsen effusion mass-loss technique, respect... |
|
|
Pyrrole alkaloids from Bolbostemma paniculatum.
J. Asian Nat. Prod. Res. 5(3) , 159-63, (2003) Three pyrrole alkaloids were isolated from Bolbostemma paniculatum. Their structures were elucidated as 4-(2-formyl-5-methoxymethylpyrrol-1-yl)butyric acid methyl ester (1), 2-(2-formyl-5-methoxymethylpyrrol-1-yl)-3-phenylpropionic acid methyl ester (2) and a... |